Abstract
In order to screen the Catalpa plant with high antioxidant activity and confirm the corresponding active fractions from Catalpa ovata G. Don, C. fargesii Bur., and C. bungei C. A. Mey., total flavonoid contents and antioxidant activities of the extracts/fractions of Catalpa plant leaves were determined. The determined total flavonoid content and antioxidant activity were used as assessment criteria. Those compounds with antioxidant activity were isolated with silica gel column chromatography and ODS column chromatography. Our results showed that the total flavonoid content in C. bungei C. A. Mey. (30.07 mg/g·DW) was the highest, followed by those in C. fargesii Bur. (25.55 mg/g·DW) and C. ovata G. Don (24.96 mg/g·DW). According to the determination results of total flavonoid content and antioxidant activity in 3 clones of leaves of C. bungei C. A. Mey., the total flavonoid content and antioxidant activity in crude extracts from C. bungei C. A. Mey. 6 (CA6) leaves were the highest. Moreover, the results showed that the total flavonoid content and antioxidant activities of ethyl acetate (EA) fraction in ethanol crude extracts in CA6 leaves were the highest, followed by n-butanol, petroleum ether (PE), and water fractions. Two flavonoid compounds with antioxidant activity were firstly isolated based on EA fraction. The two compounds were luteolin (1) and apigenin (2), respectively.
1. Introduction
The damage effect of free radicals on organisms can be inhibited by antioxidants and the inhibition effect has been recognized as a potential therapeutic way for the prevention and treatment of related diseases and become a hotspot in the field of preventive medicine [1]. The advantages of synthetic antioxidants include high efficiency and low cost. However, synthetic antioxidants also induced many security problems like the negative impact on enzyme systems in human beings [2]. On the contrary, natural antioxidants present high efficacy and small disadvantages. In this sense, compared with the synthetic antioxidants, it is necessary for the food industry to develop new effective and safe antioxidants from plants.
The Catalpa plants of Bignoniaceae family have about 13 species in the world and are mainly distributed in America and East Asia. Five species and 1 variant of Catalpa plants were introduced into China: C. ovata G. Don, C. bungei C. A. Mey., C. fargesii Bur., variant of C. tibetica Forrest, C. speciosa Ward., and C. fargesii f. duclouxii Dode, respectively. Previous phytochemical investigations indicated that iridoids and naphthoquinones were the main constituents of Catalpa plants [3–8]; C. ovata G. Don have showed antifungal activity [9, 10], anti-inflammatory activity [11–15], antitumor activity [7, 16–18], antioxidant activities [4, 19], and so on. However, the bioactivity of other Catalpa plants was seldom reported.
In this paper, the scavenging activity of DPPH radical and hydroxyl radicals and the reducing power of crude extracts from the leaves of C. ovata G. Don, C. fargesii Bur., and C. bungei C. A. Mey. were analyzed, in order to screen the Catalpa plant or clones with the highest antioxidant activity. Furthermore, the antioxidant activities of different polar fractions (ethyl acetate (EA), n-butanol, petroleum ether (PE) fraction, and water fraction) from ethanol crude extracts of C. bungei C. A. Mey. 6 (CA6) leaves were analyzed so as to obtain the highest antioxidant activity group. Moreover, further separation and purification against the highest antioxidant activities groups to identify the structure of compounds with antioxidant activities were performed. This study may lay a foundation of breeding Catalpa plant with strong antioxidant activity for the development of natural antioxidants. At the same time, the study may also provide a theoretical basis for searching natural antioxidant active ingredients from nature as resources of development of new drugs and health care.
2. Materials and Methods
2.1. General
Melting points were determined with a digital melting-point apparatus and were uncorrected. Electrospray ion trap mass spectrometry (ESI-MS) was carried out with Bruker ESI-TRAP Esquire 6000 plus mass spectrometry instrument. Nuclear magnetic resonance spectra (NMR) were recorded on a Bruker Avance III 500 MHz instrument in DMSO-d6 with tetramethylsilane (TMS) as the internal standard. Analytical thin-layer chromatography (TLC) was performed with silica gel plates and silica gel 60 GF254 (Qingdao Haiyang Chemical Co., Ltd.).
2.2. Plant Material and Chemicals
Leaves of C. ovata G. Don and C. fargesii Bur. were collected from Yangling of Shaanxi Province, China (East longitude 108° 08′, latitude 34° 27′, 440 m above sea level). Leaves of C. bungei C. A. Mey. were acquired from Tianshui in Gansu Province, China (East longitude 105° 41′, latitude 34° 14′, 1131 m above sea level). Chosen three plant materials were widely distributed in China and their leaves were collected in November 2013. A certain amount of healthy leaves with similar foliar age was collected. Leaves were heated for 20 min in an oven at 90°C immediately after collection for deactivation of enzymes and then dried at 60°C. After crushing, the leave samples were filtered with a 20-mesh sieve and then stored.
1,1-Diphenyl-2-picrylhydrazyl (DPPH), Folin-Ciocalteu reagents, and rutin (>99%) were obtained from Sigma (St. Louis, USA). 2-Deoxy-D-ribose was obtained from Aladdin (Shanghai, China). Butylated hydroxyanisole (BHA) was analytical reagents obtained from Bodi (Tianjin, China). All the reagents and solvents were of reagent grade or purified according to standard methods before use.
2.3. Determination of Total Flavonoids Contents
Total flavonoid content was determined according to the method reported by Zhishen et al. [20] with some modifications. Each sample (1 mL) was added into a 10 mL test tube, and then 3 mL methanol and 5% NaNO2 (0.3 mL) were added after 6 min. Then, 0.3 mL 10% Al(NO3)3 was added. After 6 min, 4 mL 1.0 M NaOH and 0.4 mL methanol were added, and the mixture stood alone at room temperature (RT) for 15 minutes. At last, it was measured against methanol as a blank at 510 nm. With the solution of rutin (0.50–4.00 mg/mL) as the standard, a calibration curve was plotted to calculate the content of total flavonoids.
2.4. DPPH Radical Scavenging Activity Assay
The DPPH radical scavenging activity assay was carried out according to the method reported by Brand-Williams et al. [21] with some modifications. Each sample (1 mL) was mixed with 3 mL 0.2 mM DPPH ethanol solutions. Then, 4 mL ethanol was added and incubated at RT in dark for 30 min. The absorbance was measured with spectrophotometer at 517 nm. The DPPH scavenging activity of each sample was calculated according to (1). The concentration of sample or standard antioxidant for the 50% DPPH scavenging (IC50) was also calculated. The value of IC50 was opposite to the DPPH radical scavenging activity of samples. The lower IC50 value indicates the higher DPPH radical scavenging activity. Consider
| (1) |
Note that A c = the absorbance of the control; A s = the absorbance of the sample solution.
2.5. Hydroxyl Radical Scavenging Activity Assay
Hydroxyl radical scavenging activity assay was performed according to the previously reported method [22]. Each sample (200 μL) was mixed with the mixture of 100 μL FeSO4 (10 mM), 100 μL EDTA (10 mM), 500 μL a-deoxyribose (10 mM), and 900 μL sodium phosphate buffer (0.1 M, pH 7.4). Then, 200 μL H2O2 (10 mM) was added and incubated for 1 h at 37°C. The reaction was terminated through the addition of 1.0 mL 2.8% TCA (m/v) and 1.0 mL 1.0% TBA (m/v) and incubated in boiling water for 15 min. After cooling to RT and centrifugation at 3000 r/min (TGL-16, China) for 5 min, the absorbance of the supernatant was measured at 532 nm. The hydroxyl radical scavenging activity of each sample was calculated according to (2). The concentration of sample or standard antioxidant for the 50% hydroxyl radical scavenging (IC50) was also calculated. Consider
| (2) |
Note that A c = the absorbance of the control; A s = the absorbance of the sample solution.
2.6. Reducing Power Assay
Reducing power assay was carried out according to the method reported by Oyaizu [23]. Each sample (0.5 mL) was mixed with 0.5 mL sodium phosphate buffer (0.2 M, pH 6.6) and 0.5 mL 1% potassium ferricyanide and incubated for 20 min at 50°C. Then, the mixture was cooled rapidly and 0.5 mL 10% TCA was added. After mixing and centrifugation at 3000 r/min for 20 min, 1 mL supernatant was mixed with 1.0 mL distilled water and 0.2 mL 0.1% ferric chloride and then incubated for 10 min. The absorbance was detected at 700 nm. The reducing power of samples was indicated by the percentage of the activity of 0.1 mg/mL of ascorbic acid.
2.7. Extraction and Isolation
Each treated sample (5 g) was extracted with 50 mL 80% ethanol-water solvent (v/v) at 80°C for 1 h and extraction was performed for three times. After filtration, the filtrates of total extracts were dried at 45°C under the reduced pressure. The concentrated extracts were dissolved with distilled water to the final volume of 50 mL and stored at 4°C for analysis.
CA6 leave (100 g) sample was firstly extracted and concentrated according to the method mentioned above. Then, the concentrated extracts were dispersed in 500 mL distilled water and further extracted with 500 mL of PE EA and n-butanol for 5–8 times. Each fraction was dried and dissolved with distilled water at a stock concentration of 1.0 mg/mL for the determination of total flavonoid content and antioxidant activity analysis.
The extracts with the strongest antioxidant were subjected to silica gel column chromatography with the mobile phase of the mixture of chloroform : methanol (150 : 1 to 5 : 1). Through the classification by TLC, the antioxidant activity of each fraction was determined. The fraction with the higher antioxidant activity was separated and purified. The final products were compounds 1 and 2.
2.8. Statistical Analysis
All experiment data were expressed as mean ± SD of three experiments. Statistical analysis of ANOVA for variance and SNK test was performed with SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
3.1. Total Flavonoid Content and Antioxidant Activity of Catalpa Plants
In this study, the total flavonoid contents of crude extracts from the leaves of C. ovata G. Don, C. fargesii Bur., and C. bungei C. A. Mey. were determined and the antioxidant activity of these extracts by investigating the reducing power, DPPH radical scavenging activity, and hydroxyl radical scavenging activity was analyzed.
As shown in Table 1, the total flavonoid contents of the leaves from different Catalpa plants were significantly different (P < 0.05). Among these Catalpa plants samples, leavesof C. bungei C. A. Mey. showed the highest total flavonoid content (40.45 mg/g·DW). C. fargesii Bur. ranked the second position (32.52 mg/g·DW). The total flavonoid contents in the leaves of C. ovata G. Don were the lowest (29.08 mg/g·DW). DPPH radical scavenging activity and hydroxyl radical scavenging activity could be indicated by the value of IC50; the larger the value is, the lower its activity is. The crude extracts in the leaves of C. bungei C. A. Mey. showed the strongest reducing power, DPPH radical scavenging activity, and hydroxyl radical scavenging activity, followed by C. fargesii Bur. and C. ovata G. Don. The antioxidant activity of Catalpa plant was significantly higher than others, which may relate to the structures and contents of theantioxidative activities compounds.
Table 1.
The total flavonoid contents and antioxidant activity of crude extracts from the leaves of Catalpa plants.
| Total flavonoids mg/g·DW | Reducing power % |
DPPH radical scavenging activity IC50 mg/mL |
Hydroxyl radical scavenging activity IC50 mg/mL |
|
|---|---|---|---|---|
| C. ovata G. Don | 29.08 ± 0.157c | 21.65 ± 0.080c | 2.35 ± 0.009a | 0.58 ± 0.011a |
| C. fargesii Bur. | 32.52 ± 0.102b | 24.05 ± 0.105b | 2.01 ± 0.006b | 0.13 ± 0.001b |
| C. bungei C. A. Mey. | 40.45 ± 0.143a | 34.10 ± 0.100a | 1.73 ± 0.003c | 0.11 ± 0.001c |
ANOVA. Different letters within each column indicate the statistical difference according to post hoc comparison (Student Newman Keuls) at P < 0.05.
3.2. Total Flavonoid Content and Antioxidant Activity of Clones of C. bungei C. A. Mey
The antioxidant activity of C. bungei C. A. Mey. (CA) is higher than those in C. ovata G. Don and C. fargesii Bur. Three clones of CA with relative strong antioxidant efficiency have been chosen as the experimental materials based on the early study (in press). And the antioxidant efficiency of 3 clones of CA (CA2, CA5, and CA6) was evaluated by the investigation of the total flavonoid content, reducing power, hydroxyl radical scavenging activity, and DPPH radical scavenging activity of crude extracts from plant leaves.
As shown in Table 2, the total flavonoid content and antioxidant activity of crude extracts from the 3 clones of the leaves of CA were significantly different (P < 0.05). Among the 3 clones of CA, CA6 showed the highest total flavonoids content (46.29 mg/g·DW), reducing power (41.61%), hydroxyl radical scavenging activity (IC50, 0.08 mg/mL), and DPPH radical scavenging activity (IC50, 0.98 mg/mL). However, CA5 showed the lowest total flavonoid content (32.17 mg/g·DW), reducing power (26.58%), hydroxyl radical scavenging activity (IC50, 0.15 mg/mL), and DPPH radical scavenging activity (IC50, 2.23 mg/mL).
Table 2.
The total flavonoid contents and antioxidant activity of crude extract from leaves of clones of C. bungei C. A. Mey.
| Clones | Total flavonoids mg/g·DW | Reducing power % | DPPH radical scavenging activity IC50 mg/mL | Hydroxyl radical scavenging activity IC50 mg/mL |
|---|---|---|---|---|
| CA2 | 34.62 ± 0.319b | 27.49 ± 0.080b | 1.97 ± 0.004b | 0.10 ± 0.001b |
| CA5 | 32.17 ± 0.353c | 26.58 ± 0.139c | 2.23 ± 0.008a | 0.15 ± 0.001a |
| CA6 | 46.29 ± 0.245a | 41.61 ± 0.080a | 0.98 ± 0.008c | 0.08 ± 0.001c |
ANOVA. Different letters within each column indicate the statistical difference according to post hoc comparison (Student Newman Keuls) at P < 0.05.
The total flavonoid content and antioxidant activity of crude extracts from C. ovate G. Don, C. fargesii Bur., and the 3 clones of leaves of CA were significantly different. This result indicated that there was a big difference among different kinds and different clones of the Catalpa plant in total flavonoid content and antioxidant activities. The result was also consistent with previous reports on the difference of total flavonoid content and antioxidant activity of apples [24, 25] and pomegranate [26] resulting from fruit varieties and producing area. The accumulations of secondary metabolites in plants were mainly influenced by genetic adaptation capability and ecological environment, such as temperature, moisture, illumination, and altitude [27]. For example, plants in the high altitude area can synthesize flavonoids with the strong absorption in the ultraviolet region to reduce the UV radiation damage. Therefore, the CA6 in high altitude area, because of the strong ultraviolet radiation, has the high flavonoid content and antioxidant activity.
3.3. Total Flavonoid Content and Antioxidant Activity of Various Fractions of CA6
With CA6 leaves, the reducing power, hydroxyl radical scavenging activity, and DPPH radical scavenging activity of different polar fractions (PE, EA, n-butanol, and water fraction) extracted from ethanol crude extracts of CA6 leaves were further investigated, with BHT as positive control.
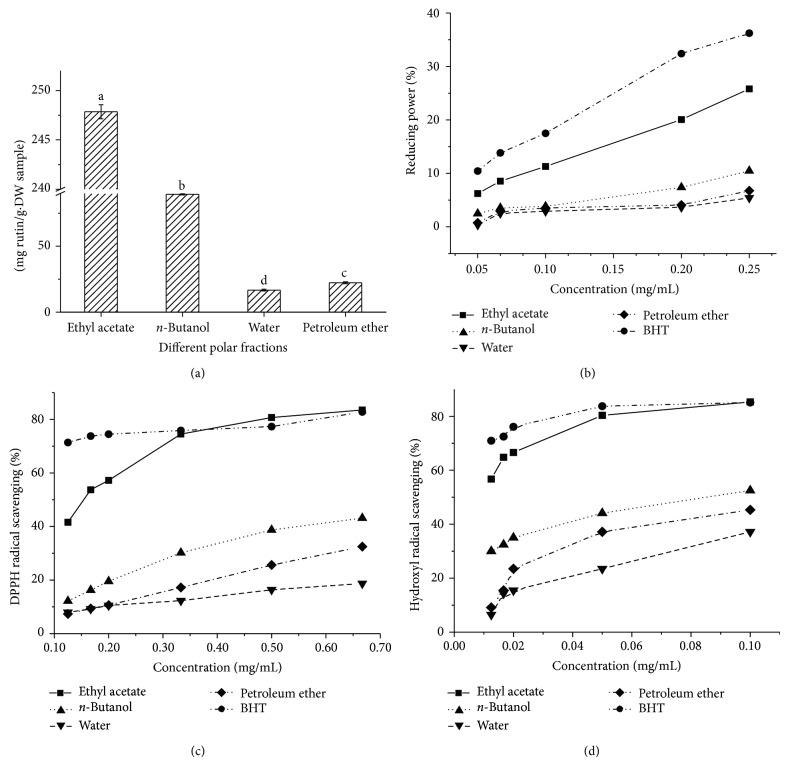
As shown in Figure 1(a), the total flavonoid content of different polar fractions was significantly different (P < 0.05). From nonpolar PE to and polar water phase, all fractions contain flavonoid compounds. The total flavonoid content of EA fraction (247.85 mg/g·DW) was significantly higher than that of n-butanol (89.31 mg/g·DW) and PE fraction (22.24 mg/g·DW). The total flavonoid content of water phase (16.71 mg/g·DW) was significantly lower than those of others. Thus, the flavonoid compounds of CA6 leaves mainly existed in EA fraction.
Figure 1.
Total flavonoid content and antioxidant activity of various fractions of CA6. (a) Total flavonoid content; (b) reducing power; (c) DPPH radical scavenging activity; (d) hydroxyl radical scavenging activity.
As shown in Figure 1(b), all the different polar fractions of crude extracts of CA6 leaves exhibited the reducing power in a dose-dependent manner at the concentration of 0.05–0.25 mg/mL. Among these fractions, the EA fraction showed the highest reducing power, followed by the n-butanol fraction, PE fraction, and water residue.
As shown in Figure 1(c), the DPPH radical scavenging activity of different polar fractions showed a dose-dependent manner at the concentration of 0.13–0.67 mg/mL. Compared with the DPPH radical scavenging activity of BHT, different polar fractions displayed the significantly weaker scavenging activity at the lower concentration of 0.13–0.33 mg/mL. Only EA fraction shows similar activity to BHT at the higher concentration of 0.50–0.67 mg/mL. Among the four fractions, the EA fraction presented the highest activity at all the tested concentrations, followed by n-butanol fraction and PE fraction. The aqueous residue showed the lowest DPPH radical scavenging activity.
The hydroxyl radical scavenging activity of different polar fractions of crude extracts of CA6 leaves was further determined. The results showed that all the different polar fractions of crude extracts of CA6 leaves exhibited hydroxyl radical scavenging activity at the concentration of 0.01–0.10 mg/mL and showed a dose-dependent effect (Figure 1(d)). The EA fraction presented the highest hydroxyl radical scavenging activity, followed by n-butanol fraction, PE fraction, and aqueous residue. Compared with the hydroxyl radical scavenging activity of BHT, the EA fraction has a slightly lower hydroxyl radicals scavenging activity when its concentration was less than 0.05 mg/mL, but the EA fraction revealed similar activity to BHT when the concentration was more than 0.10 mg/mL, which indicates the strong hydroxyl radical scavenging activity.
The EA fraction of different polar fraction derived from crude extracts of the CA6 leaves presented the highest antioxidant activity and flavonoid content under all the tested concentrations. The result was consistent with previous reports on the antioxidant activity and flavonoid content of EA fraction derived from crude extracts of Dianthus superbus [28], Toona sinensis M. Roem. [29], and Ruellia tuberosa [30]. Especially, the CA6 presents the equal antioxidant activity with BHT at a certain concentration and may be a potential resource for development of natural antioxidants.
3.4. Isolation and Identification of the Components with Antioxidant Activity
The EA fraction with the highest antioxidant activity was isolated by silica gel column chromatography, and the eluent was divided into seven fractions (Fractions 1–7) according to the polarity sequence of solvent (Figure 2). Then, the DPPH radical scavenging activity of the seven fractions with the same concentration BHT as the positive control was determined (Figure 3). The DPPH radical scavenging activity of seven fractions and BHT was significantly different (P < 0.05) at the concentration of 0.10 mg/mL. Among the seven fractions, the DPPH radical scavenging activity of Fr. 5 was the highest, followed by Fr. 7.
Figure 2.
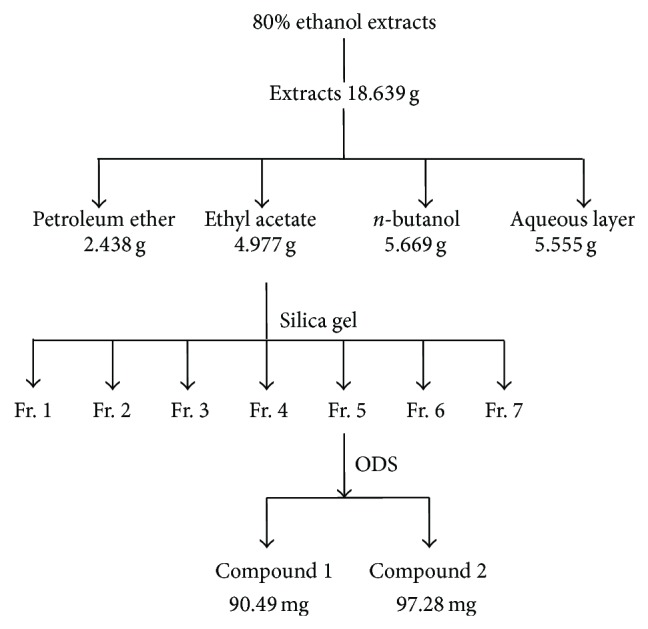
The process of extraction, isolation, and fractionation of bioactive compounds from CA6.
Figure 3.
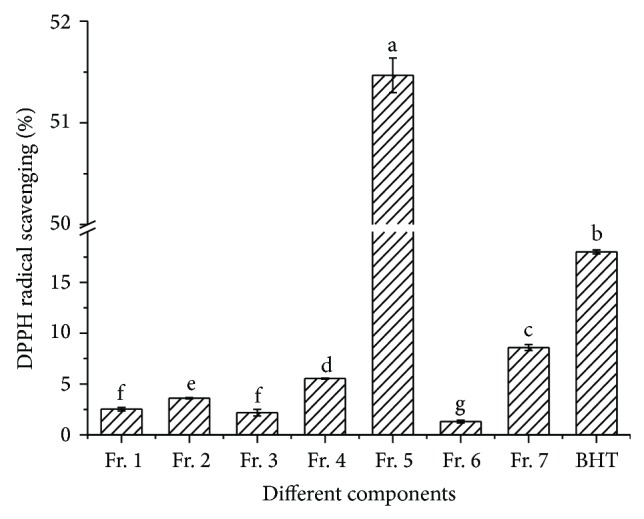
DPPH radical scavenging activity of 7 subfractions.
Two compounds 1 and 2 were isolated from Fr. 5 by the ODS column chromatography. The DPPH radical scavenging activity and reducing power of compounds 1 and 2 at the concentration of 0.10 mg/mL were further investigated. The DPPH radical scavenging activity and reducing power of compound 1 were 84.04% and 45.58%, respectively, which were higher than those in compound 2 and BHT at the same concentration.
Data for 1. CAS: 128475-17-2, yellow solid, m.p. 328–330°C; 1H NMR (400 MHz, DMSO-d 6) δ: 12.99 (s, 1H, H-5), 9.84 (s, 3H, H-7, 3′, 4′), 7.40–7.44 (m, 2H, H-2′, 6′), 6.88 (d, J = 8.0 Hz, 1H, H-5′), 6.68 (s, 1H, H-3), 6.45 (d, J = 2.0 Hz, 1H, H-8), 6.19 (d, J = 2.0 Hz, 1H, H-6), 13C NMR (100 MHz, DMSO-d 6) δ: 182.1 (C-4), 164.6 (C-7), 164.3 (C-2), 161.9 (C-9), 157.7 (C-5), 150.1 (C-4′), 146.1 (C-3′), 121.9 (C-6′), 119.4 (C-1′), 116.4 (C-5′), 113.8 (C-2′), 104.1 (C-3), 103.3 (C-10), 99.2 (C-6), 94.3 (C-8); MS (ESI-TRAP), m/z (%): 285.22 ([M-H]+, 100). The data of compound 1 was in accordance with the result reported by Pettit et al. [31]. Therefore, compound 1 should be luteolin.
Data for 2. CAS: 128475-17-2, yellow solid, m.p. 345–350°C; 1H NMR (400 MHz, DMSO-d 6) δ: 12.98 (s, 1H, H-5), 10.60 (s, 2H, H-7, 4′), 7.92 (d, J = 8.8 Hz, 2H, H-2′, 6′), 6.92 (d, J = 8.8 Hz, 2H, H-3′, 5′), 6.79 (s, 1H, H-3), 6.49 (d, J = 2.0 Hz, 1H, H-8), 6.20 (d, J = 2.0 Hz, 1H, H-6); 13C NMR (100 MHz, DMSO-d 6) δ: 182.2 (C-4), 164.6 (C-2), 164.1 (C-7), 161.9 (C-4′), 161.6 (C-9), 157.7 (C-5), 128.9 (C-2′, 6′), 121.6 (C-1′), 116.4 (C-3′, 5′), 104.1 (C-10), 103.3 (C-3), 99.2 (C-6), 94.3 (C-8); MS (ESI-TRAP), m/z (%): 269.20 ([M-H]+, 100). The data of compound 2 was in accordance with the result reported by Ding et al. [32]. Therefore, compound 2 should be apigenin.
The structures of luteolin (1) and apigenin (2) (Figure 4) indicate that they are all flavonoid compounds. The difference between in luteolin (1) and apigenin (2) was only hydroxyl group, but the antioxidant activities of two compounds were significantly different. More hydroxyl groups indicate the stronger antioxidant activities. Besides, both C-3′ and C-4′ of luteolin (1) have hydroxyl groups that can form the adjacent two phenolic hydroxyl groups' structure, thus resulting in the higher DPPH radical scavenging activity and reducing power. In conclusion, the number of hydroxyl groups and the structure of adjacent two phenolic hydroxyl groups are the foundation of the highly active flavonoid compounds.
Figure 4.
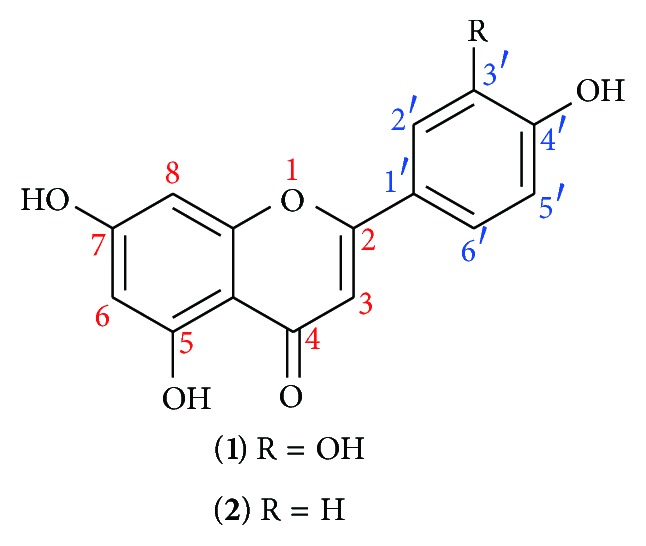
Chemical structures of luteolin (1) and apigenin (2).
4. Conclusions
In this paper, the antioxidant activity of the C. ovata G. Don, C. bungei C. A. Mey., C. fargesii Bur., and the 3 clones of C. bungei C. A. Mey. was investigated. The results demonstrated that CA6 presented the highest antioxidant activity and the main material with antioxidation ability was medium polar compounds. Moreover, the two flavonoid compounds (luteolin 1; apigenin 2) from the EA fraction of the CA6 were isolated. This study provided a new plant source for the extraction of luteolin (1) and apigenin (2). A new way for development and utilization of Catalpa plants was also rendered.
Acknowledgments
This work was supported by National Forestry Public Welfare Industry Research Project (no. 201204603) and National Natural Science Foundation of China (no. 31170274).
Conflict of Interests
There is no conflict of interests regarding the publication of the paper.
References
- 1.Bardia A., Tleyjeh I. M., Cerhan J. R., et al. Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: Systematic review and meta-analysis. Mayo Clinic Proceedings. 2008;83(1):23–34. doi: 10.4065/83.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Sun B., Fukuhara M. Effects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoids on the activation of mutagens and drug-metabolizing enzymes in mice. Toxicology. 1997;122(1-2):61–72. doi: 10.1016/S0300-483X(97)00078-4. [DOI] [PubMed] [Google Scholar]
- 3.Zan L. X., Zhao Y., Sun J. W. Extraction and content determination of catapol in the seeds of C. fargesii Bur. Jorunal of Chinese Medicinal Materials. 2005;28(1):26–27. [Google Scholar]
- 4.Machida K., Hishinuma E., Kikuchi M. Studies on the constituents of Catalpa species. IX. Iridoids from the fallen leaves of Catalpa ovata G. DON. Chemical & Pharmaceutical Bulletin. 2004;52(5):618–621. doi: 10.1248/cpb.52.618. [DOI] [PubMed] [Google Scholar]
- 5.Machida K., Ando M., Yaoita Y., Kakuda R., Kikuchi M. Studies on the constituents of Catalpa species. VI. Monoterpene glycosides from the fallen leaves of Catalpa ovata G. Don. Chemical and Pharmaceutical Bulletin. 2001;49(6):732–736. doi: 10.1248/cpb.49.732. [DOI] [PubMed] [Google Scholar]
- 6.Machida K., Ogawa M., Kikuchi M. Studies on the constituents of Catalpa species. II. Iridoids from Catalpae fructus. Chemical and Pharmaceutical Bulletin. 1998;46(6):1056–1057. doi: 10.1248/cpb.46.1056. [DOI] [Google Scholar]
- 7.Fujiwara A., Mori T., Iida A., et al. Antitumor-promoting naphthoquinones from Catalpa ovata. Journal of Natural Products. 1998;61(5):629–632. doi: 10.1021/np9800147. [DOI] [PubMed] [Google Scholar]
- 8.Kanai E., Machida K., Kikuchi M. Studies on the constituents of Catalpa species. I. Iridoids from Catalpae fructus. Chemical and Pharmaceutical Bulletin. 1996;44(8):1607–1609. doi: 10.1248/cpb.44.1607. [DOI] [Google Scholar]
- 9.Cho J. Y., Kim H. Y., Choi G. J. Dehydro-alpha-lapachone isolated from Catalpa ovata stems: activity against plant pathogenic fungi. Pest Management Science. 2006;62(5):414–418. doi: 10.1002/ps.1180. [DOI] [PubMed] [Google Scholar]
- 10.Kuk J.-H., Ma S.-J., Moon J.-H., Kim K.-Y., Choi S.-H., Park K.-H. Antibacterial and antifungal activities of a naphthoquinone derivative isolated from the fruits of Catalpa ovata G. DON. Journal of Microbiology and Biotechnology. 2002;12(5):858–863. [Google Scholar]
- 11.Yang G., Choi C.-H., Lee K., Lee M., Ham I., Choi H.-Y. Effects of Catalpa ovata stem bark on atopic dermatitis-like skin lesions in NC/Nga mice. Journal of Ethnopharmacology. 2013;145(2):416–423. doi: 10.1016/j.jep.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Park B. M., Hong S. S., Lee C., Lee M. S., Kang S. J., Shin Y. S., Jung J.-K., Hong J. T., Kim Y., Lee M. K., Hwang B. Y. Naphthoquinones from catalpa ovata and their inhibitory effects on the production of nitric oxide. Archives of Pharmacal Research. 2010;33(3):381–385. doi: 10.1007/s12272-010-0306-2. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.-W., Choi S.-C., Choi E.-Y., et al. Catalposide, a compound isolated from Catalpa ovata, attenuates induction of intestinal epithelial proinflammatory gene expression and reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice. Inflammatory Bowel Diseases. 2004;10(5):564–572. doi: 10.1097/00054725-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Pae H. O., Oh G. S., Choi B. M., Shin S., Chai K. Y., Oh H., Kim J. M., Kim H. J., Jang S. I., Chung H. T. Inhibitory effects of the stem bark of Catalpa ovata G. Don. (Bignoniaceae) on the productions of tumor necrosis factor-α and nitric oxide by the lipopolisaccharide-stimulated RAW 264.7 macrophages. Journal of Ethnopharmacology. 2003;88(2-3):287–291. doi: 10.1016/S0378-8741(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 15.An S. J., Pae H. O., Oh G. S., Choi B. M., Jeong S., Jang S. I., Oh H., Kwon T. O., Song C. E., Chung H. T. Inhibition of TNF-α, IL-1β, and IL-6 productions and NF-κB activation in lipopolysaccharide-activated RAW 264.7 macrophages by catalposide, an iridoid glycoside isolated from Catalpa ovata G. Don (Bignoniaceae) International Immunopharmacology. 2002;2(8):1173–1181. doi: 10.1016/S1567-5769(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 16.Oh C.-H., Kim N.-S., Yang J. H., et al. Effects of isolated compounds from catalpa ovata on the t cell-mediated immune responses and proliferation of leukemic cells. Archives of Pharmacal Research. 2010;33(4):545–550. doi: 10.1007/s12272-010-0408-x. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki R., Yasui Y., Kohno H., Miyamoto S., Hosokawa M., Miyashita K., Tanaka T. Catalpa seed oil rich in 9t,11t,13c-conjugatedlinolenic acid suppresses the development of colonic aberrant crypt foci induced by azoxymethane in rats. Oncology Reports. 2006;16(5):989–996. [PubMed] [Google Scholar]
- 18.Muñoz-Mingarro D., Acero N., Llinares F., et al. Biological activity of extracts from Catalpa bignonioides Walt. (Bignoniaceae) Journal of Ethnopharmacology. 2003;87(2-3):163–167. doi: 10.1016/S0378-8741(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 19.Dvorská M., Žemlička M., Muselík J., Karafiátová J., Suchý V. Antioxidant activity of Catalpa bignonioides. Fitoterapia. 2007;78(6):437–439. doi: 10.1016/j.fitote.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 1999;64(4):555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 21.Brand-Williams W., Cuvelier M. E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 22.Ren J., Zhao M., Shi J., Wang J., Jiang Y., Cui C., Kakuda Y., Xue S. J. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chemistry. 2008;108(2):727–736. doi: 10.1016/j.foodchem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Oyaizu M. Studies on products of the browning reaction. Antioxidative activities of browning reaction products prepared from glucosamine. Japanese Journal of Nutrition. 1986;44(6):307–315. [Google Scholar]
- 24.Łata B., Trampczynska A., Paczesna J. Cultivar variation in apple peel and whole fruit phenolic composition. Scientia Horticulturae. 2009;121(2):176–181. doi: 10.1016/j.scienta.2009.01.038. [DOI] [Google Scholar]
- 25.Khanizadeh S., Tsao R., Rekika D., Yang R., Charles M. T., Vasantha Rupasinghe H. P. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. Journal of Food Composition and Analysis. 2008;21(5):396–401. doi: 10.1016/j.jfca.2008.03.004. [DOI] [Google Scholar]
- 26.Mousavinejad G., Emam-Djomeh Z., Rezaei K., Khodaparast M. H. H. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chemistry. 2009;115(4):1274–1278. doi: 10.1016/j.foodchem.2009.01.044. [DOI] [Google Scholar]
- 27.Dong J., Ma X., Wei Q., Peng S., Zhang S. Effects of growing location on the contents of secondary metabolites in the leaves of four selected superior clones of Eucommia ulmoides. Industrial Crops and Products. 2011;34(3):1607–1614. doi: 10.1016/j.indcrop.2011.06.007. [DOI] [Google Scholar]
- 28.Yu J.-O., Liao Z.-X., Lei J.-C., Hu X.-M. Antioxidant and cytotoxic activities of various fractions of ethanol extract of Dianthus superbus . Food Chemistry. 2007;104(3):1215–1219. doi: 10.1016/j.foodchem.2007.01.039. [DOI] [Google Scholar]
- 29.Jiang S.-H., Wang C.-L., Chen Z.-Q., et al. Antioxidant properties of the extract and subfractions from old leaves of Toona sinensis roem (meliaceae) Journal of Food Biochemistry. 2009;33(3):425–441. doi: 10.1111/j.1745-4514.2009.00226.x. [DOI] [Google Scholar]
- 30.Chen F.-A., Wu A.-B., Shieh P., Kuo D.-H., Hsieh C.-Y. Evaluation of the antioxidant activity of Ruellia tuberosa . Food Chemistry. 2006;94(1):14–18. doi: 10.1016/j.foodchem.2004.09.046. [DOI] [Google Scholar]
- 31.Pettit G. R., Meng Y., Stevenson C. A., Doubek D. L., Knight J. C., Cichacz Z., Pettit R. K., Chapuis J.-C., Schmidt J. M. Isolation and structure of palstatin from the amazon tree Hymeneae palustris. Journal of Natural Products. 2003;66(2):259–262. doi: 10.1021/np020231e. [DOI] [PubMed] [Google Scholar]
- 32.Ding H.-Y., Chen Y.-Y., Chang W.-L., Lin H.-C. Flavonoids from the flowers of Pueraria lobata. Journal of the Chinese Chemical Society. 2004;51(6):1425–1428. [Google Scholar]