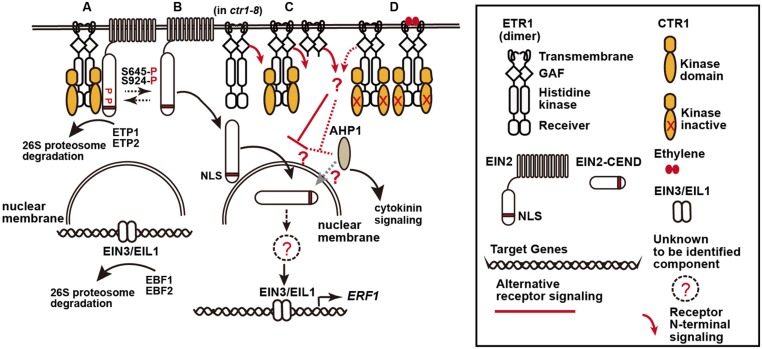
FIGURE 1.
A model of the ethylene receptor signaling via the C- and N-termini of ethylene receptor. (A) Ethylene receptor C-terminal signaling, here represented by ETHYLENE RESPONSE1 (ETR1), is mediated via the histidine kinase domain to CTR1 to phosphorylate EIN2 residues S645 and S924, and EIN2 is retained at the endoplasmic reticulum for degradation mediated by the F-box proteins EIN2 TARGETING PROTEIN1 (ETP1) and ETP2. Ethylene signaling mediated by EIN2 to the nuclear EIN3/EIL1 is inhibited, and EIN3 and EIL1 undergo degradation mediated by the F-box proteins EIN3 BINDING F-BOX PROTEIN1 (EBF1) and EBF2. (B) “Default” ethylene signaling by EIN2. Without the receptors (in the receptor quintuple mutant), EIN2-mediated ethylene signaling fully occurs and the mutants produce an extremely strong constitutive ethylene response phenotype. With the receptors, a portion of EIN2 could be underphosphorylated, presumably because of interconversion between the phosphorylated and underphosphorylated state or incomplete phosphorylation by CTR1, and cleaved to potentially activate ethylene signaling. (C,D) Ethylene signaling suppression by the receptor N-terminus: (C) With the receptors, the “default” ethylene signaling mediated by EIN2 can be repressed in part by the receptor N-terminal signaling (red line), regardless of CTR1 docking. (D) Ethylene binding or docking of a kinase-inactive ctr1 at the receptors inhibits the receptor C-terminal signaling, and the cleaved EIN2 C-terminus is translocated to the nucleus to induce EIN3/EIL1-directed expression of ethylene response genes. The N-terminal signaling is also inhibited (red dotted line), and the inhibition of EIN2-mediated signaling is largely alleviated. Components involved in the receptor N-terminal signaling remain to be identified. AHP1 and EIN2 are proposed to be involved in the CTR1-independent pathway because of their interaction with ETR1, and AHP1 also mediates cytokinin signaling.