Abstract
Aim
To study the effects of the tumor necrosis factor alpha inhibitor adalimumab on rabbit retina after injection into the vitreous body.
Methods
Forty-eight rabbits of mixed strain (9–12 months old, weighing ≈ 3.5 kg) were randomized into four groups. Adalimumab was injected at one of two concentrations (1.25 mg or 2.5 mg) into the eyes of two groups, and balanced salt solution into the eyes of the third group. The fourth group acted as controls. Full-field electroretinography (ffERG) was performed before injection and 1 and 6 weeks post-injection. At 6 weeks post-injection the rabbits were euthanized and the sectioned retinas were studied. Retinal histology was studied with hematoxylin–eosin staining. Immunohistochemical analysis was performed on rods, cones, rod bipolar cells, horizontal cells, amacrine cells and Müller cells.
Results
No significant difference in ffERG amplitudes or implicit times was observed between the four groups at any time point. Histological and immunohistochemical findings were similar in all groups.
Conclusions
Injection of adalimumab into the vitreous body of healthy rabbits, at doses up to 2.5 mg, does not appear to be toxic to the rabbit retina.
Keywords: Adalimumab, drug toxicity, electrophysiology, histopathology, intravitreal injection, rabbit retina, retinal function, tumor necrosis factor-alpha inhibitor
INTRODUCTION
Adalimumab is a fully humanized monoclonal antibody against the cytokine tumor necrosis factor alpha (TNFα). Tumor necrosis factors are a group of cytokines that play a role in systemic inflammation stimulating the acute phase reaction. TNFα is produced by activated macrophages, CD4+ lymphocytes and natural killer cells. It plays a pivotal role in the initiation and perpetuation of inflammation, it induces apoptosis and it inhibits tumor genesis and viral replication.1–5 However, the persistent production of TNFα, as in autoimmune inflammatory diseases, including uveitis, is associated with significant tissue damage.2 In uveitis, activated retinal microglia, Müller cells and retinal pigment epithelial cells generate TNFα.4 Blocking TNFα has proven very successful in the treatment of autoimmune conditions such as rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, psoriatic arthritis and juvenile idiopathic arthritis.2 ,5 In uveitis, levels of TNFα are raised in the aqeous humor and in ocular tissues.4 Injection of TNFα into the vitreous body results in uveitis, leading to disruption of the blood–retina barrier and neovascularization.6 It has been found in experimental autoimmune uveitis, that the clinical onset of disease and histologic damage was supressed by neutralising TNFα activity.4
Systemic treatment with TNFα inhibiting substances has been used successfully, as an off-label alternative to traditional immunosuppressive treatment in the management of severe and refractory forms of uveitis such as Behçet‘s disease, sarcoidosis, juvenile uveitis and idiopathic uveitis.3 ,7–15
TNFα has been implicated in other ocular conditions as well. It has been identified in fibrovascular membranes in proliferative diabetic retinopathy, its messenger RNA (mRNA) has been found in subretinal fluid and in the vitreous body of patients with rhegmatous retinal detachment in combination with proliferative vitroretinopathy.16–18 Increased levels of its soluble receptors have also been reported in the vitreous body of patients with retinal detachment and proliferative vitroretinopathy.19 It has been suggested that TNFα is involved in the neurodegenerative process of glaucoma and in post-ischemic conditions.20–22 In addition, TNFα may play a role in the pathogenesis of choroidal neovascularization and neovascular age-related macular degeneration.3 ,23–27
Of the five TNFα inhibitors presently on the market infliximab, etanercept and adalimumab are most frequently used, followed by golimumab and certolizumab. Infliximab and adalimumab have shown promising results in the treatment of uveitis.28
Adalimumab, infliximab, and etanercept have similar intrinsic binding properties for soluble TNF and for membrane bound TNF.29
However, infliximab is more immunogenic and anaphylactic reactions have been reported in association with systemic treatment and retinotoxic reactions after intravitreal injection.3 ,12 ,30 ,31 Etanercept has been reported to be less efficient in treating uveitis than adalimumab and infliximab and some studies have shown that it may even induce uveitis.9 ,12 ,32 ,33–35 Since adalimumab seems to be well tolerated when administered systemically, showing good clinical effect on uveitis we consider it important to investigate the safety of adalimumab when injected into the vitreous body using a rabbit model.12 ,34 Retinal toxicity was assessed using ffERG and at the end of the study the retinas were examined histologically and immunohistochemically.
MATERIALS AND METHODS
Animals
Forty-eight pigmented Swedish lop eared rabbits of a mixed strain (aged 9–12 months, body weight ≈3.5 kg) were included in the study. The rabbits were randomized into four groups of 12 animals. Groups 1 and 2 received an intravitreal injection into the right eye of 0.05 mL adalimumab at concentrations of 1.25 mg and 2.5 mg, respectively. The third group was given an intravitreal injection of balanced salt solution (BSS), and the final group, receiving no injection served as controls. FfERG was performed before injection and at 1 and 6 weeks post-injection. Six weeks after the injection and after the final ffERG measurement, the rabbits were euthanized by an intravenous overdose of penthobarbital and the retinas were sectioned and processed for hematoxylin and eosin staining, and for immunohistochemical analysis. Complete data could not be obtained from five rabbits: one died after baseline ERG, belonging to the control group, and four died after the second ffERG measurement (two from the group given 1.25 adalimumab, one from the group given 2.5 adalimumab, and one from the BSS-injected group).
The rabbits were housed in separate cages with standard nutrition and water ad libitum. The study was conducted following the approval of the Ethical Committee for Animal Research at Lund University, Sweden and all experimental procedures were performed in compliance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research.
Intravitreal Injections
The right eye was anesthesized with 0.4% oxibuprocain eye drops (Chauvin, Bausch and Lomb, Stockholm, Sweden), and a lid speculum was placed under the eyelid. A commercial solution of adalimumab, Humira® (Abbot Scandinavia AB, Solna, Sweden) with a concentration of 50 mg/mL was used. This was diluted with 0.09% saline to a concentration of 2.5 mg/0.05 mL and 1.25 mg/0.05 mL. The volume injected was 0.05 mL of 1.25 mg adalimumab, 2.5 mg adalimumab or of BSS, 1.5 mm behind limbus into the vitreous body using a 30-gauge needle. Care was taken to avoid touching the lens with the needle.
Full-Field Electroretinogram
Standardized ffERG, slightly modified for the rabbit by placing the ground electrode on the neck and by not using light adaptation preceding the 30-Hz flicker stimulation, were recorded with a Nicolet analysis system (Nicolet Biomedical Instruments, Madison, WI) as described previously.36 All rabbits were examined with ffERG three times: at baseline (before injection) as well as one and 6 weeks after injection. During the examination the rabbits were sedated by an intramuscular injection of (0.1 mL/kg) Hypnorm (fentanylcitrate 0.315 mg/mL and fluanisone 10 mg/mL). The ffERG examinations were conducted according to standards of the International Society for Clinical Electrophysiology of Vision.37 The right eye was tested after maximal pupil dilation using a topical application of 1% cyclopentolate hydrochloride (Chauvin, Bausch and Lomb, Stockholm, Sweden), and after 30 min of dark adaptation. A Burian–Allen bipolar ERG contact lens electrode was applied to the topically anesthetized cornea together with a subcutaneous ground electrode on the neck. Responses were obtained with a wide-band filter (−3 dB at 1 Hz and 500 Hz) following stimulation with single full-field flashes (30 µs) and with 30-Hz flickering white light. The referred luminance of the different light stimuli has been measured on the light reflected from a Ganzfeld sphere (350 Linear/Log Optometer, Graseby Optronics, calibrated by UDT Instuments, Baltimore, MD). Each recording was repeated at each stimulus intensity, to ensure reproducibility (i.e. two consecutive identical responses were obtained). Responses to the following stimulations were recorded:
the dark adapted response to a single flash of dim white light, with an integrated luminance 0.81 cd s/m2, representing the isolated rod-mediated response.
the dark adapted response to a single flash of white light, with an integrated luminance 3.93 cd s/m2, representing the combined rod- and cone- mediated response.
response to 30-Hz flickering white light, with an integrated luminance 0.81 cd s/m2, with no background illumination, averaged over 20 sweeps, representing the isolated cone mediated response.
the light-adapted response to a single flash with an integrated luminance 3.93 cd s/m2, with background illumination, representing the isolated cone mediated response.
Tissue Preparation
After the final ffERG examination, the rabbits were euthanized and the right eye was immediately enucleated and fixed for 30 min in 0.1 M Sørensen’s phosphate buffer, at pH 7.4, containing 4% paraformaldehyde (Merck, Darmstadt, Germany). The bulbs were then transected at the ora serrata and the anterior and posterior segments separated. The posterior segment was postfixed in the same fixative for 3.5 h, at 4 °C. The tissue was rinsed and cryoprotected by transferring it stepwise through two solutions containing 10% and 20% sucrose in Sørensen’s buffer. The posterior segment was divided into two by a vertical incision from the superior to the inferior retinal margins through the center of the optic disc. The posterior segments were then embedded in Yazulla medium (30% egg albumen and 3% gelatin in water) and sectioned (12 µm) in a cryostat (−21 °C). The sections were mounted on chrome alum coated slides, air dried and stored at −20 °C until analyzed.
IMMUNOHISTOCHEMISTRY
Analysis of Glial Fibrillary Acidic Protein, Protein Kinase C Alpha, Calbindin, Parvalbumin and Rhodopsin
The sections were thawed and rinsed in 0.1 M sodium phosphate-buffered saline (PBS), pH 7.2 with 0.25% Triton X-100 (PBST). Bovine serum albumin (1%) was added in PBST to dilute the primary and secondary antibodies. The sections were then incubated with the primary antibodies (Table 1) for 16–18 h at 4 °C.38–43 After 1 h at room temperature, the slides were rinsed in PBST and further incubated with the appropriate fluorescent secondary antibody (1:200), for 45 min in darkness. After rinsing, the slides were mounted in a custom-made anti-fading mounting medium. The same labeling procedure without the primary antibody was performed to obtain negative control samples. To confirm their efficacy, the primary antibodies were used on sections that have previously stained positive for glial fibrillary acidic protein (GFAP), protein kinase C alpha (PKCα), calbindin, parvalbumin or rhodopsin, respectively. Specimens were also stained with hematoxylin and eosin. The slides were examined using immunofluorescence imaging and photographed using a digital camera (Nikon Eclipse 800). The same exposure time and aperture were used for all samples, and the same magnification (×40) was used for all photographs. No image processing was applied to the photographs.
TABLE 1.
Antibodies used for histochemical labeling of retinal sections.
| Marker | Concentration | Target | Poly/mono clonal | References | Source |
|---|---|---|---|---|---|
| Antibody against protein kinase alpha MC5 | 1:200 | Rod bipolar cells | mono | Kostinaho et al.37 and Wood et al.38 | Nordic BioSite, Täby, Sweden |
| Antibody against glial fibrillary acidic protein | 1:200 | Astrocytes and activated Müller cells | mono | Lewis P et al.39 | Chemicon International, Billerica, MA |
| Antibody against parvalbumin | 1:1000 | Amacrine cells | mono | Casini et al.40 | Sigma, St Louis, MO |
| Antibody against rhodopsin | 1:100 | Rod photoreceptors | mono | Mc Kenzie et al.41 | Kind gift of Prof. RS Molday, Vancouver Canada |
| Antibody against calbinin D-28K | 1:200 | Horizontal cells | mono | Massey et al.42 | Sigma, St Louis, MO |
| Biotinylated peanut agglutinin | 1:500 | Cone photoreceptors | mono | Blanks et al.43 | Vector Laboratories inc., Burlingame, CA |
Analysis of Peanut Agglutinin
The sections were thawed and rinsed in 0.1 M sodium PBS, pH 7.2. They were first incubated for 45 min in biotinylated peanut agglutinin (PNA) (Table 1) in PBS (1:500) and then in rhodamine-conjugated streptavidin in PBS (1:1000) for 30 min.44 After rinsing with PBS, the slides were mounted in the same custom-made anti-fading mounting medium. The same labeling procedure without the primary antibody was performed to obtain negative controls. PNA staining was classified as either positive or negative for each individual. As a positive control, PNA labeling was performed on normal adult rabbit retina. The slides were examined using immunofluorescence imaging and photographed using the digital camera. No image processing was applied.
Statistical Analysis
SPSS Statistics 20 (IBM Corporation, Somers, NY) was used for statistical analysis of the results obtained with ffERG. The four groups were compared using ANOVA Mixed Model analysis. The ffERG parameters at 1 and 6 weeks post-injection were compared, using the pre-injection value as a covariate (Table 2). An overall analysis of the treatment effect at both post injection time points simultaneously was performed using a mixed model analysis with repeated measurements. In that model, the measurements at the two time points were assumed to be dependent and different covariance structures were tried out to model the dependence. However, the different structures gave similar results and finally an AR(1) (first order autoregressive) model was chosen.
TABLE 2.
Results of the ANOVA Mixed Model analysis comparing ERG amplitudes in the 4 groups at 1 and 6 weeks post-injection.
| ERG response | ANOVA mixed model overall treatment effect |
|---|---|
| Response to dim white light single flash (WND2) b-wave amplitude | p = 0.917 |
| Response to Dark- adapted single white flash (W1.0) a-wave amplitude | p = 0.659 |
| Response to dark adapted single white flash (W1.0) b-wave amplitude | p = 0.832 |
| Response to 30-Hz flicker b-wave amplitude | p = 0.095 |
| Implicit time for 30-Hz flicker b-wave | p = 0.450 |
| Response to light-adapted single white flash(BOnW1.0) b-wave | p = 0.418 |
No significant differences were found between groups.
For the statistical analysis of the histology results, PKCα-labeled rod-bipolar cells were counted using the method previously described by Kjellström et al., i.e. the number of stained perikarya and axons/terminals per window on photographs obtained under the microscope with the ×40 objective in one representative retinal section.45 The scores for the perikarya and axons/terminals were compared separately. The investigator was blinded to the identity of the retinal sections of PKCα-labeled cells. Comparative statistical analyses were carried out using the Kruskal–Wallis one-way analysis of variance (ANOVA), which is a non-parametric alternative to the ANOVA. Descriptive analyses were performed without further quantification for the other antibodies. Sections of the central retina were evaluated with regard to GFAP labeling. Sections of the central and peripheral retina were analyzed to determine the degree of labeling for calbindin, rhodopsin, PNA and parvalbumin.
RESULTS
ERG Findings
Descriptive statistics are shown in a box plot in Figure 1. The analysis of the effect of treatment, at 1 and 6 weeks post-injection combined using the ANOVA Mixed Model analysis with repeated measurements, showed no significant differences in ERG amplitudes, or in the implicit times for the b-wave in response to 30-Hz flicker, between the four groups, at any time point (Table 2).
FIGURE 1.
Descriptive statistics for the ffERG measurements, in the form of a box plot giving the median and range. (A) The isolated rod-mediated retinal response to dim white light (WND2), (B) the total dark-adapted retinal response (a-wave amplitude) to single-flash of white light (W1.0), (C) the total dark-adapted retinal response (b-wave amplitude) to the single-flash of white light (W1.0), (D) the isolated cone-mediated dark-adapted retinal response (b-wave amplitude) to 30 Hz flickering white light (Flicker), (E) the implicit time of the b-wave response to 30-Hz flickering white light, (F) the isolated cone-mediated retinal response (b-wave amplitude) to the single-flash of white light. Data for the different measuring occasions (baseline, 1 week after and 6 weeks after injection) are indicated by different colors. The ordinate indicates the amplitude in µV, and the abscissa the group (no injection, injection of BSS, and 1.25 mg or 2.5 mg adalimumab). Circles and asterisks represent outliers and extreme values, values that are 1.5 or 3 times the height of the box outside the either end of the box, respectively.
Clinical Observations
Ophthalmoscopic examination and dissection of the right eye from all rabbits showed that the retinas were attached and there were no cataracts.
Histological Findings
Hematoxylin- and eosin- stained slides showed normal retinal architecture without signs of vacuoles or edema in any of the animal groups (Figure 2).
FIGURE 2.
Retinal sections, stained with hematoxylin and eosin, from one rabbit in each group, 6 weeks after injection, showing no significant difference between the groups. (A) Controls; (B) 0.05 ml balanced salt solution; (C) 1.25 mg adalimumab; (D) 2.5 mg adalimumab. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclerar layer; OS, outer segments of photoreceptors. Scalebar = 30 µm.
Histochemical Findings
PKCα-labeled bipolar cells were seen in the retinal sections of all four groups, with perikarya located in the outer part of the inner nuclear layer and axons terminating in the inner plexiform layer (Figure 3).The immunolabeling was evenly distributed over the entire cell, perikarya as well as axons and terminals. The PKCα antibody also labeled the outer segments of the photoreceptors in all sections. Kruskal–Wallis one-way analysis revealed no significant difference between the groups (p = 0.123for perikarya, and p = 0.087 for axons). Strong immunolabeling for PNA showed intact inner and outer segments of cone photoreceptors in all four groups. Moderate labeling was also seen in the cone cell perikarya in the outer nuclear layer and their axons, terminating in the outer plexiform layer. No difference was observed in the number of labeled cells for any of the groups.
FIGURE 3.
Retinal sections from one rabbit from each group, 6 weeks after injection, stained with PKC antibodies, showing rod bipolar cells. (A) No injection; (B) balanced salt solution; (C) adalimumab 1.25 mg; (D) adalimumab 2.5 mg. Comparative statistical analysis was carried out using Kruskal–Wallis one way analysis. No significant differences were found between the groups. Scalebar = 30 µm.
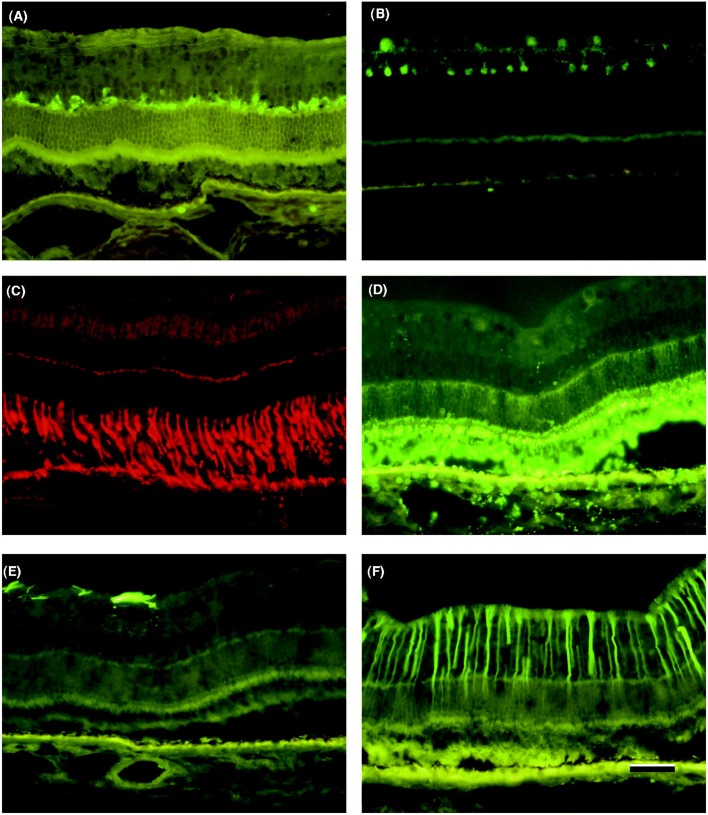
Retinal sections labeled for GFAP showed normal labeling of astrocytes in the central part of the retina. Two to four central sections from each of the four groups showed labeling of Müller cells with the anti-GFAP antibody (4/11 in Group 1, 2/11 in Group 2, 3/10 in Group 3, and 2/11 in Group 4). Parvalbumin-antibody-labeled amacrine cells were seen on both sides of the inner plexiform layer. Intense labeling for rhodopsin was seen in the outer segments of the rods. The horizontal cells with perikarya in the inner nuclear layer and axons extending horizontally in the outer plexiform layer were well labeled by the anti-calbindin antibody. In some cases, the anti-calbindin antibody also labeled cone bipolar cells in the inner nuclear layer. No differences in labeling were seen with antibodies raised against parvalbumin, rhodopsin, Müller cells, or calbindin in retinal sections from any of the four groups. Examples of the images obtained with these antibodies are shown in Figures 3 and 4.
FIGURE 4.
Retinal sections showing one example of each histological staining used in this study. No significant differences were found between the groups. In each of the four groups 2–4 rabbits showed positive staining for glial fibrillary protein (GFAP). (A) Staining for calbindin-labeled horizontal cells in the outer plexiform layer, (B) staining for parvalbumin- labeled amacrine cells on both sides of the inner plexiform layer, (C) staining for peanut agglutinin (PNA) labeled cone photoreceptors, (D) staining for rhodopsin- labeled rod photoreceptors, (E) staining for glial fibrillary protein (GFAP) showing no labeling of Müller cells but labeling of microglia, (F) staining for glial fibrillary protein (GFAP) showing positive labeling of Müller cells. Scalebar = 30 µm.
DISCUSSION
The results of this study provide strong evidence that the injection of adalimumab, into the vitreous body of rabbit eyes, is safe in a dose up to 2.5 mg. FfERG amplitudes were not affected, indicating no functional disturbances as a result of the injected substance, and normal retinal architecture was seen after staining retinal sections with hematoxylin and eosin. Immunohistochemical analysis of retinal sections showed no difference in the labeling of rods, cones, rod bipolar cells, horizontal or amacrine cells between the groups.
Only pigmented and no albino rabbits were used, as the ocular response to toxic substances seem to differ between animals with pigmented eyes and animals without pigment in their eyes. The probable mechanism behind this is that melanin binds substances and decreases the peak concentration. Since patients usually have pigmented eyes, using pigmented rabbits is more relevant.46
As adalimumab is a fully humanized monoclonal antibody, some of the immunogenic reactions observed in patients treated with infliximab, a chimeric mouse/human anti TNFα antibody, can be avoided and co-treatment with methotrexate is not an obligatory part of the therapeutic regimen.11 ,12 ,32 ,30 Neither does it induce uveitis, as has been reported with etanercept.11 ,12 ,33 The recommended route of administration of adalimumab is by sub-cutaneous injection. Injection into the vitreous body can provide a drug reservoir, ensure that an adequate drug concentration is delivered and avoid adverse systemic effects. Since the introduction of anti-vascular endothelial growth factor therapies for neovascular macular degeneration, injection into the vitreous body has become a very common procedure. In a study using tracers, Peyman et al. reported that when a substance was injected into the vitreous body it traveled across the retina and stopped at the junctional complexes of the retinal pigment epithelium. Similarly a substance injected into the systemic circulation passed through the choriocapillaris and Bruch‘s membrane and stopped at the junctional complexes in the retinal epithelium. Therefore, intravitreal administration is a very efficient route of achieving a high intraocular concentration.47 The adverse effects associated with intravitreal injections are similar among different agents. The most common are infectuous endophthalmitis (0.019–1.6%), sterile intraocular inflammation (1.4–2.9 %), regmatous retinal detachment (0–0.67%), intraocular pressure elevation, ocular hemorrhage, mostly subconjunctival.48 Cataract formation has also been reported. None of these occured in this study.
As the blood–retina barrier is circumvented by intravitreal injection, toxicological testing using the same route will be necessary. The appropriate therapeutic dose of adalimumab for intravitreal use has not been determined. The single dose injected in other studies on intravitreal injection of adalimumab in rabbits varies between 0.5 mg and 10 mg.49–51 We wanted to inject a dose that we thought could neutralise TNFα in the ocular tissues and fluids for an extended period of time, and since we wanted to exclude or detect toxicity we did not want to inject a dose a dose too low to reach toxic levels. Finally, we reasoned as follows:
The eye constitutes about 0.012% of total bodyweight, assuming that the average weight of a human being is 65 kg and that the eye weighs 7.5 g. The recommended starting dose of adalimumab is 40 mg injected sub-cutaneously every 14 d. Dosing according to weight would give a dose into the eye of 0.5 mg adalimumab. As the goal is to avoid repetitive intra-vitreal injections, finally two empiric doses of 1.25/0.05 mL or 2.5/0.05 mL adalimumab were chosen. Considering that the volume of the vitreous cavity of the rabbit eye is smaller than that of the human eye (1.5 versus 4.0 mL), the dose of adalimumab that is safe for the human eye may be higher than the 2.5 mg injected in this study. Our study was not designed to determine therapeutically relevant doses, but toxic/non-toxic doses.
Few other studies on the safety of intravitreal use of adalimumab have been carried out. Manzano et al. conducted two studies on the safety of intravitreal injection of adalimumab on rabbits: a pilot study followed by an extended study. In both studies ffERG was performed at baseline and 2 weeks after injection. From the pilot study they concluded that a single dose of 0.5 mg was safe, while 1.0 mg resulted in a reduction in ffERG amplitudes and inflammatory reactions in two of the three included rabbits. Retinal necrosis was found in one rabbit. In one of these three rabbits the lens capsule had been pierced by the needle during injection, which in itself could lead to an inflammatory reaction.51 The results of the pilot study were not confirmed in the more extensive study. There was a decrease in the amplitude of the a-wave and in the implicit time of the b-wave, in one group, however, the conclusion was no toxicity with an intravitreal dose up to 5 mg in any of the six rabbits included in this group. Injection of 10 mg adalimumab led to a decrease in a-wave amplitude in the photopic response and a mild inflammatory reaction in the anterior chamber. Staining with hematoxylin and eosin indicated preserved retinal architecture. No pathology was seen with the electron microscopy in doses up to 5 mg adalimumab, while for the group that received 10 mg the results were inconclusive.50 Tsilimbaris et al. injected 0.5 or 5 mg adalimumab into the eyes of groups of eight rabbits using the left eye as a control. FfERG was performed at baseline and 2 weeks post-injection. The rabbits were euthanized after 2 weeks and the retina was studied under light and electron microscope. Injection of adalimumab in the two chosen doses was concluded to be safe.49
In the above studies, the time span from injection to euthanasia were shorter, the immunohistochemical examination less detailed and the animal groups smaller. In this study, the histology was more elaborate and the time span from injection to the final ERG and histology examination longer, 6 instead of 2 weeks. This allows longer contact between the substance and the retina and more closely resembles a clinical situation. Two studies do, however, provide examination with electron microscopy.49 ,50
As TNFα inhibitors have been successfully used systemically in an off-label manner for treatment of several forms of non-infectuous uveitis treatment by injection directly into the vitreous body is an appealing idea.1 ,7–15 Reflecting this, a few minor studies have been carried out on patients, testing the safety and efficacy of intravitreal injection of infliximab and adalimumab. The patients included in these studies have had long-standing, recalcitrant uveitic macular edema and neovascular macular degeneration, sub-optimally responsive to vascular endothelial growth factor blocking substances.32 ,52 ,53 ,30 ,54 Infliximab, but not adalimumab, was found to cause uveitis in one study and both substances were found ineffective in four of the studies.32 ,52 ,53 ,30 Since the safety of intravitreal injection of adalimumab has not been confirmed before, only longstanding cases, not responsive to conventional treatment, have been subjected to this novel administration route of this agent. As evidence on the safety of this treatment accumulates, patients with these diseases may be treated earlier with local adalimumab, increasing the probability for success.
Findings from some studies have indicated that TNFα may play a role in other ophthalmological conditions, such as glaucoma, retinal detachment and ocular neovascularizations20 ,55–58 and TNFα inhibition may offer a therapy for treating these conditions as well.
During the study five rabbits died. One died after the base ERG, before any injection had been made. Four rabbits died after the second ERG. From these one belonged to the BSS injected group, two to the group injected with 1.25 mg/50 µL and one to the 2.5 mg/50 µL group. Since only one of the five belonged to the group injected with the highest concentration of adalimumab, we consider it improbable that this caused by adalimumab toxicity. Also, the method of injection and the ERG procedure, including sedation, was the same as our group has been using in several previous studies using rabbits of this strain.
In conclusion, the results of this study show that a single intravitreal injection of adalimumab into the rabbit eye at a concentration up to 2.5 mg has no negative effects on retinal function or on retinal histology. Intravitreal administration of adalimumab could, therefore, be a useful therapeutic tool in the treatment of uveitis, avoiding adverse systemic side effects.
Acknowledgements
We would like to thank Boel Nilsson, Karin Arnér and Christer Månsson for their skillful technical assistance.
Footnotes
Declaration of interest This study was supported by grants from Synskadade i fd Malmöhus län, Stiftelsen Olle Engkvist, Stiftelsen Synfrämjandets Forsknings-fond, Kronprinsessans Margaretas Arbetsnämnd, Torsten and Ragnar Söderbergs Stiftelser, the Swedish Medical Research Council (project no. 2007-3385) and the Faculty of Medicine at Lund University. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.Rifkin LM, Birnbaum AD, Goldstein DA. TNF inhibitionfor ophthalmic indications:current status and outlook. BioDrugs. 2013;27:347–357. doi: 10.1007/s40259-013-0022-9. [DOI] [PubMed] [Google Scholar]
- 2.Khera TK, Dick AD, Nicholson LB. Mechanisms of TNFα regulation in uveitis: focus on RNA-binding proteins. Prog Retin Eye Res. 2011;30:610–621. doi: 10.1016/j.preteyeres.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Mirshahi A, Hoehn R, Lorenz K, Kramann C, Baatz H. Anti-tumor necrosis factor alpha for retinal diseases:current knowledge and future concepts. J Ophthalmic Vis Res. 2012;7:39–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Dick AD, Forrester JV, Liversidge J, Cope AP. The role of tumour necrosisi factor(TNF-α) in experimental autoimmune uveoretinitis (EAU) Prog Retin Eye Res. 2004;23:617–637. doi: 10.1016/j.preteyeres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Malaviya AP, Ostör AJ. Rheumatoid arthritis and the era of biologic therapy. Inflammopharmacol. 2012;20:59–69. doi: 10.1007/s10787-012-0123-y. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum JT, Howes EL, Rubin R, Samples JR. Ocular inflammatory effects of intravitreally-injected tumour necrosis factor. Am J Pathol. 1988;133:47–53. [PMC free article] [PubMed] [Google Scholar]
- 7.Mushtaq B, Saeed T, Situnayake RD, Murray PI. Adalimumab for sight-threatening uveitis in Behçet‘s disease. Eye (Lond) 2007;21:824–825. doi: 10.1038/sj.eye.6702352. [DOI] [PubMed] [Google Scholar]
- 8.Galor A, Perez VL, Hammel JP, Lowder CY. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology. 2006;113:2317–2323. doi: 10.1016/j.ophtha.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 9.Erckens RJ, Mostard RL, Wijnen PA, Schouten JS, Drent M. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2012;250:713–720. doi: 10.1007/s00417-011-1844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Accorinti M, Pirraglia MP, Paroli MP, Priori R, Conti F, Pivetti-Pezzi P. Infliximab Treatment for ocular and extraocular Behçet‘s disease. Jpn J Ophthalmol. 2007;51:191–196. doi: 10.1007/s10384-006-0425-y. [DOI] [PubMed] [Google Scholar]
- 11.Biester S, Deuter C, Michels H, Haefner R, Kuemmerle-Deschner J, Doycheva D, Zierhut M. Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol. 2007;91:319–324. doi: 10.1136/bjo.2006.103721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bawazeer A, Raffa LH, Nizamuddin SH. Clinical experience with adalimumab in the treatment of ocular Behçet disease. Ocul Immunol Inflamm. 2010;18:226–232. doi: 10.3109/09273948.2010.483314. [DOI] [PubMed] [Google Scholar]
- 13.van Laar JA, Missotten T, van Daele PL, Jamnitski A, Baarsma GS, van Hagen PM. Adalimumab: a new modality for Behçet‘s disease? Ann Rheum Dis. 2007;66:565–566. doi: 10.1136/ard.2006.064279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph A, Raj D, Dua HS, Powell PT, Lanyon PC, Powell RJ. Infliximab in the treatment of refractory posterior uveitis. Ophthalmology. 2003;110:1449–1453. doi: 10.1016/S0161-6420(03)00406-8. [DOI] [PubMed] [Google Scholar]
- 15.Foeldvari I, Nielsen S, Kümmerle-Deschner J, Espada G, Horneff G, Bica B, et al. Tumor necrosis factor alpha blocker in treatment of juvenile idiopathic arthritis associated uveitis refractory to second line agents: results of a multinational survey. J Rheumatol. 2007;34:1146–1150. [PubMed] [Google Scholar]
- 16.Limb GA, Chignell AH, Green W, LeRoy F, Dumonde DC. Distribution of TNFa and vascular adhesion molecules in fibrocellular membranes of proliferative diabetic retinopathy. Br J Opthlamol. 1996;80:168–173. doi: 10.1136/bjo.80.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Ghrably IA, Dua HS, Orr GV, Fischer D, Tighe PJ. Detection of cytokine mRNA production in infiltrating cells in proliferative vitreoretinopathy using reverse transcription polymerase chain reaction. Br J Ophthalmol. 1999;83:1296–1299. doi: 10.1136/bjo.83.11.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limb GA, Earley O, Jones SE, LeRoy F, Chignell AH, Dumonde DC. Expression of mRNA coding for TNF alpha, IL-1 beta and IL-6 by cells infiltrating retinal membranes. Graefes Arch Clin Exp Ophthalmol. 1994;232:646–651. doi: 10.1007/BF00171378. [DOI] [PubMed] [Google Scholar]
- 19.Limb GA, Hollifield RD, Webster L, Charteris DG, Chignell AH. Soluble TNF receptors in vitreoretinal proliferative disease. Invest Ophthalmol Vis Sci. 2001;42:1586–1591. [PubMed] [Google Scholar]
- 20.Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001;42:1787–1794. [PubMed] [Google Scholar]
- 21.Berger S, Savitz SI, Nijhawan S, Singh M, David J, Rosenbaum PS, Rosenbaum DM. Deleterious role of TNF-a in retinal ischemia-reperfusioin injury. Invest Opthalmol Vis Sci. 2008;49:3605–3610. doi: 10.1167/iovs.07-0817. [DOI] [PubMed] [Google Scholar]
- 22.Gesslein B, Håkansson G, Gustavsson L, Ekström P, Malmsjö M. Tumor necrosis factor and its receptors in the neuroretina and retinal vasculature after ischemia-reperfusion injury in the pig retina. Mol Vis. 2010;16:2317–2327. [PMC free article] [PubMed] [Google Scholar]
- 23.Semkova I, Muether PS, Kuebbeler M, Meyer KL, Kociok N, Joussen AM. Recruitment of blood-derived inflammatory cells mediated via tumor necrosis factor (TNF)-a receptor 1b exacerbates CNV. Invest Ophthalmol Vis Sci. 2011;52:6101–6108. doi: 10.1167/iovs.10-5996. [DOI] [PubMed] [Google Scholar]
- 24.Jasielska M, Semkova I, Shi X, Schmidt K, Karagiannis D, Kokkinou D, Mackiewicz J, et al. Differential role of tumor necrosis factor (TNF) alphareceptors in the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:3874–3883. doi: 10.1167/iovs.09-5003. [DOI] [PubMed] [Google Scholar]
- 25.Lichtlen PD, Lam T, Nork M, Streit T, Urech DM. Relative contribution of VEGF and TNF-alpha in the cynomolgus laser-induced CNV model:comparing efficacy of bevacizumab, adalimumab and ESBA105. Invest Ophthalmol Vis Sci. 2010;51:4738–4735. doi: 10.1167/iovs.09-4890. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida S, Yoshida A, Ishibashi T. Induction of IL-8, MCP-1, and bFGF by TNF-a in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefe‘s Arch Clin Exp Ophthalmol. 2004;242:409–413. doi: 10.1007/s00417-004-0874-2. [DOI] [PubMed] [Google Scholar]
- 27.Olsson JL, Courtney RJ, Mandava N. Intravitreal infliximab and choroidal neovascularization in an animal model. Arch Ophthalmol. 2007;125:1221–1224. doi: 10.1001/archopht.125.9.1221. [DOI] [PubMed] [Google Scholar]
- 28.Martel J N, Esterberg E, Nagpal A, Acharya NR. Infliximab and adalimumab for uveitis. Ocul Immunol Inflamm. 2012;20:18–20. doi: 10.3109/09273948.2011.633205. [DOI] [PubMed] [Google Scholar]
- 29.Kaymakcalan Z, Sakorafas P, Bose S, Scesney S, Xiong L, Hanzatian Karaglu D, et al. Comparisons of affinities, avidities and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol. 2008;131:308–316. doi: 10.1016/j.clim.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Giganti M, Beer PM, Lemanski N. Adverse events after intravitreal infliximab (Remicade) Retina. 2010;30:71–80. doi: 10.1097/IAE.0b013e3181bcef3b. [DOI] [PubMed] [Google Scholar]
- 31.Thalayasingam N, Isaacs JB. Anti-TNF therapy. Best Pract Res Clin Rheumatol. 2011;25:549–567. doi: 10.1016/j.berh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Wu L, Arevalo F, Hernandez-Bogantes E, Regatieri C, Rocca JA, Farah ME. Intravitreal tumor necrosis factor alpha inhibitors for neovascular age related degeneration suboptimally responsive to anti-vascular endotheial growth factor agents: a pilot study from the pan-American collaborative retina study group. J Ocul Pharmacol Ther. 2013;29:366–371. doi: 10.1089/jop.2012.0203. [DOI] [PubMed] [Google Scholar]
- 33.Lim LL, Frauenfelder FW, Rosenbaum JT. Do tumor necrosis inhibitors cause uveitis, a registry-based study. Arthritis Rheum. 2007;56:3248–3252. doi: 10.1002/art.22918. [DOI] [PubMed] [Google Scholar]
- 34.Mansour AM. Adalimumab in the therapy of uveitis in childhood. Brit J Ophthalmol. 2007;91:274–276. doi: 10.1136/bjo.2006.108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy AR, Backhouse OC. Does etanercept induce uveitis? Brit J Ophthalmol. 2002;87:1–11. doi: 10.1136/bjo.87.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gjörloff K, Andréasson S, Ehinger B. Standardized full-field electroretinography in rabbits. Doc Ophthalmol. 2004;109:163–168. doi: 10.1007/s10633-004-3924-5. [DOI] [PubMed] [Google Scholar]
- 37.Marmor M, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2008;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 38.Koistinaho J, Sagar SM. Localization of protein kinase C subspecies in the rabbit retina. Neurosci Lett. 1994;177:15–18. doi: 10.1016/0304-3940(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 39.Wood JPM, McCord RJ, Osborne NN. Retinal protein kinase C. Neurochem Int. 1997;30:119–136. doi: 10.1016/s0197-0186(96)00049-6. [DOI] [PubMed] [Google Scholar]
- 40.Lewis P, Fisher SK. Up regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003;230:263–289. doi: 10.1016/s0074-7696(03)30005-1. [DOI] [PubMed] [Google Scholar]
- 41.Casini G, Rickman DW, Brecha NC. AII amacrine cell population in the rabbit retina: identification by parvalbumin immunoreactivity. J Comp Neurol. 1995;356:132–142. doi: 10.1002/cne.903560109. [DOI] [PubMed] [Google Scholar]
- 42.MacKenzie D, Arendt A, Hargrave P, McDowell JH, Molday RS. Localization of binding sites for carboxyl terminal specific antirhodopsin monoclonal antibodies using synthetic peptides. Biochemistry. 1984;18:6544–6549. doi: 10.1021/bi00321a041. [DOI] [PubMed] [Google Scholar]
- 43.Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol. 1996;366:15–33. doi: 10.1002/(SICI)1096-9861(19960226)366:1<15::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 44.Blanks J, Johnson LV. Specific binding of peanut lectin to a class of retinal photoreceptor cells. Invest Ophthalmol Vis Sci. 1982;25:546–557. [PubMed] [Google Scholar]
- 45.Kjellström U, Bruun A, Ghosh F, Andréasson S, Ponjavic V. Dose-related changes in retinal function and PKC-alpha expression in rabbits on vigabatrin medication. Graefe‘s Arch Clin Exp Ophthalmol. 2009;247:1057–1067. doi: 10.1007/s00417-009-1093-7. [DOI] [PubMed] [Google Scholar]
- 46.Perlman I. Testing retinal toxicity of drugs in animals using electrophysiological and morpholpgical techniques. Doc Opthalmol. 2009;118:3–28. doi: 10.1007/s10633-008-9153-6. [DOI] [PubMed] [Google Scholar]
- 47.Peyman GA, Conway MD, Fiscella R. Interaction of intravitreal combination drugs and the effect on the targeted site. J Ocul Pharmacol Ther. 2009;25:387–394. doi: 10.1089/jop.2009.0027. [DOI] [PubMed] [Google Scholar]
- 48.Ghasemi Falavarjani K, Ngyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye. 2013;27:787–794. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsilimbaris M, Diakonis VF, Naoumidi I, Charisis S, Kritikos I, Chatzithanasis G, et al. Evaluation of potential retinal toxicity of adalimumab (Humira) Graefes Arch Clin Exp Ophthalmol. 2009;247:1119–1125. doi: 10.1007/s00417-009-1065-y. [DOI] [PubMed] [Google Scholar]
- 50.Manzano PR, Peyman GA, Carvounis PE, Damico FM, Aguiar RG, Ioshimoto GL, et al. Toxicity of high-dose intravitreal adalimumab (humira) in the rabbit. J Ocul Pharmacol Ther. 2011;27:327–331. doi: 10.1089/jop.2010.0174. [DOI] [PubMed] [Google Scholar]
- 51.Manzano RP, Peyman GA, Carvounis PE, Kivilcim M, Khan P, Chevez-Barrios P, et al. Ocular toxicity of intra-vitreous adalimumab (Humira) in the rabbit. Graefes Arch Clin Exp Ophthalmol. 2008;246:907–911. doi: 10.1007/s00417-008-0765-z. [DOI] [PubMed] [Google Scholar]
- 52.Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP. Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am J Ophthalmol. 2009;147:825–830. doi: 10.1016/j.ajo.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Androudi S, Tsironi E, Kalogeropoulos C, Theodoridou A, Brazitikos P. Intravitreal adalimumab for refractory uveitis-related macular edema. Ophthalmology. 2010;117:1612–1616. doi: 10.1016/j.ophtha.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Farvardin M, Afarid M, Sharzad S. Long-term effects of intravitreal infliximab for treatment of sight-threatening chronic noninfectious uveitis. J Ocul Pharmacol Ther. 2012;28:628–631. doi: 10.1089/jop.2011.0199. [DOI] [PubMed] [Google Scholar]
- 55.Nakasawa T, Kayama M, Ryu M, Kunikata H, Watanabe R, Yasuda M, et al. Tumor necrosis factor–a mediates photoreceptor death in a rodent model of retinal detachment. Invest Ophthalmol Vis Sci. 2011;52:1384–1391. doi: 10.1167/iovs.10-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakasawa T, Matsurbata A, Noda K, Hisatomi T, She H, Skondra D, et al. Characterization of cytokine response to retinal detachment in rats. Mol Vis. 2006;12:867–878. [PubMed] [Google Scholar]
- 57.De Oliveira Dias JR, Büchele Rodrigues E, Maia M, Magalhaes O, Marcondes Penha F, Farah ME. Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol. 2011;95:1631–1637. doi: 10.1136/bjo.2010.186361. [DOI] [PubMed] [Google Scholar]
- 58.Giraudo E, Primo L, Audero E, Gerber HP, Koolwijk P, Soker S, et al. Tumor necrosis factor–alpha regulates expression of endothelial growth factor receptor-2 and its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem. 1998;273:22128–22135. doi: 10.1074/jbc.273.34.22128. [DOI] [PubMed] [Google Scholar]