Figure 4.
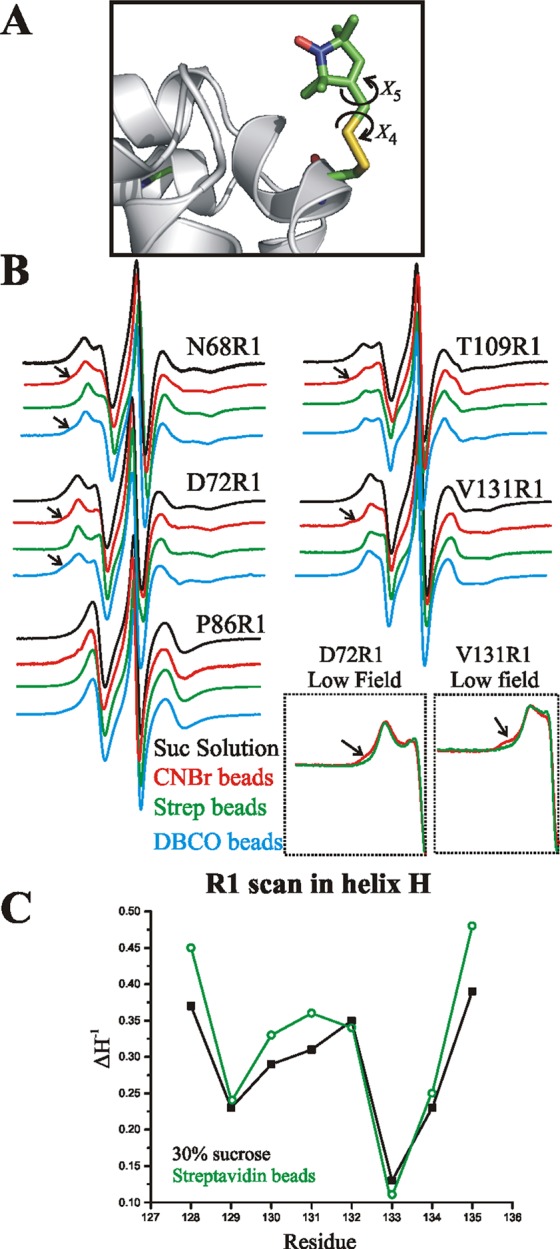
Monitoring structure and flexibility of T4L site-specifically attached to a solid support with CW lineshape analysis. (A) Model of R1 side chain in a helix showing points of internal flexibility about the last two dihedral angles (X4/X5; see text). (B) EPR spectra of the indicated sites recorded in 30% w/w sucrose (black), tethered to CNBr-Sepharose (red), or tethered site-specifically to streptavidin–Sepharose by scheme 3 (green) or by covalent attachment according to scheme 1 (cyan). The arrows identify new relatively immobile states observed after attachment. Insets: The low field lines of the spectra of 72R1 and 131R1 tethered to streptavidin and CNBr-Sepharose are magnified to reveal more clearly the spectral component corresponding to an immobile nitroxide. T4L 68R1 and 72R1 were tethered via site 131p-AzF, 86R1 via 44p-AzF, and 109R1 via 68p-AzF. (C) Plot of the inverse central line width (ΔH–1) along the sequence 128–135 in helix H; the proteins were tethered via 44p-AcF.
