Figure 2.
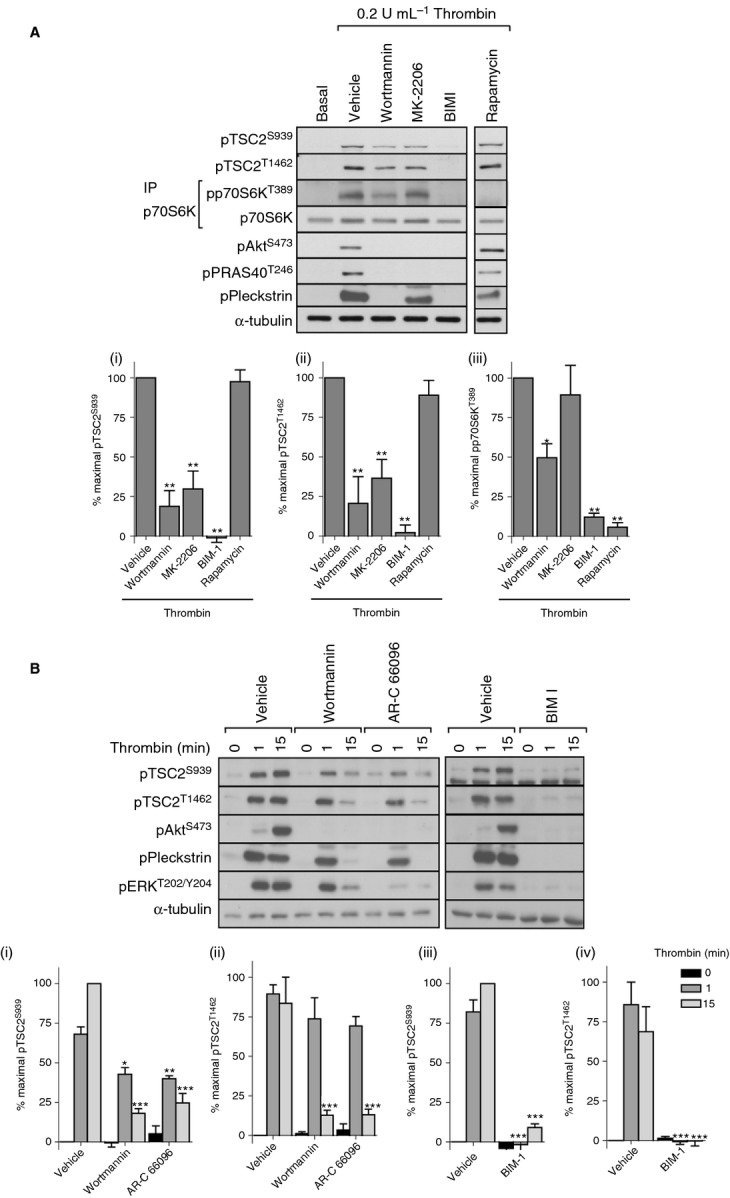
Akt-independent, PKC-dependent phosphorylation of p70S6K. Washed platelets were incubated with vehicle (0.2% DMSO), wortmannin (100 nmol L−1), MK-2206 (1 μmol L−1), BIM I (10 μmol L−1), or rapamycin (200 nmol L−1) for 15 min before stimulation with thrombin (0.2 U mL−1) for 15 min (A) or with vehicle (0.2% DMSO), wortmannin (100 nmol L−1), AR-C 66096 (1 μmol L−1), or BIM I (10 μmol L−1) for 15 min before stimulation with thrombin (0.2 U mL−1) for 1 or 15 min (B). Phosphorylation of the indicated proteins was assessed by western blotting of either whole cell lysates or immunoprecipitates (IP). Membranes were reprobed for α-tubulin to confirm equal loading. The bar graphs depict quantification of pTSC2 at Ser939 and Thr1462 (ratio phosphorylated/loading control) and pp70S6K at Thr389 (ratio phosphorylated/total) expressed as a percentage of the maximal signal induced by thrombin in control conditions. *P < 0.05, **P < 0.01, ***P < 0.001, indicating a significant difference from vehicle control (A) or between vehicle control and inhibitor-treated samples at the matching time point (B). Results are expressed as mean ± SE.
