Figure 2.
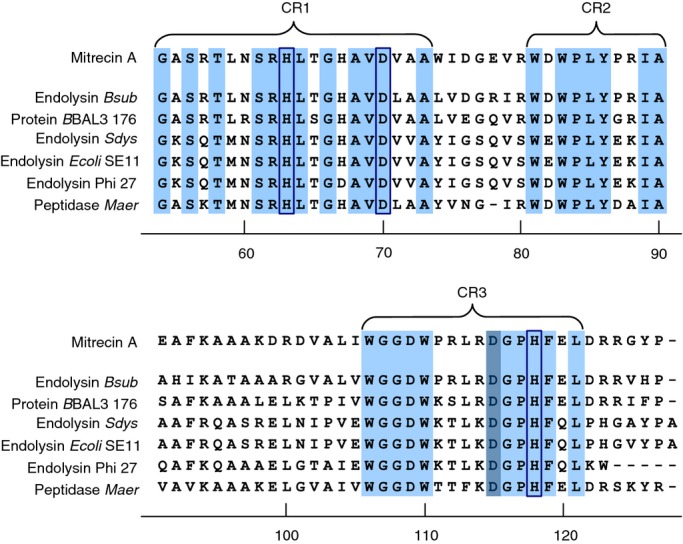
Conserved architecture of the C-terminal catalytic region of Mitrecin A as compared to other similar carboxypeptidases. C-termini of sequences with similarity to Mitrecin A (Fig. 1), identified in MEROPS Blast searches, were aligned against the Mitrecin A C-terminus using the clustalw multiple alignment tool. Conserved amino acid residues and motifs within the C-terminal M15 peptidase subfamily domains as aligned against Mitrecin A are shaded in light blue. Conserved region 1 (CR1), conserved region 2 (CR2), and conserved region 3 (CR3) are indicated. Histidine (H) and aspartic acid (D) residues, inferred by analysis in MEROPS to be necessary for interaction with the metal ion of the catalytic site binding pocket, are enclosed in boxes. The active site residue is shaded in dark blue.
