Abstract
Background
This study evaluated the prescribing trends of four commonly prescribed strong opioids in primary care and explored utilization in non-cancer and cancer users.
Methods
This cross-sectional study was conducted from 2000 to 2010 using the UK Clinical Practice Research Datalink. Prescriptions of buprenorphine, fentanyl, morphine and oxycodone issued to adult patients were included in this study. Opioid prescriptions issued after patients had cancer medical codes were defined as cancer-related use; otherwise, they were considered non-cancer use. Annual number of prescriptions and patients, defined daily dose (DDD/1000 inhabitants/day) and oral morphine equivalent (OMEQ) dose were measured in repeat cross-sectional estimates.
Results
In total, there were 2,672,022 prescriptions (87.8% for non-cancer) of strong opioids for 178,692 users (59.9% female, 83.9% non-cancer, mean age 67.1 ± 17.0 years) during the study period. The mean annual (DDD/1000 inhabitants/day) was higher in the non-cancer group than in the cancer group for all four opioids; morphine (0.73 ± 0.28 vs. 0.12 ± 0.04), fentanyl (0.46 ± 0.29 vs. 0.06 ± 0.24), oxycodone (0.24 ± 0.19 vs. 0.038 ± 0.028) and buprenorphine (0.23 ± 0.15 vs. 0.008 ± 0.006). The highest proportion of patients were prescribed low opioid doses (OMEQ ≤ 50 mg/day) in both non-cancer (50.3%) and cancer (39.9%) groups, followed by the dose ranks of 51–100 mg/day (26.2% vs. 28.7%), 101–200 mg/day (15.1% vs. 19.2%) and >200 mg/day (8.25% vs. 12.1%).
Conclusions
There has been a huge increase in strong opioid prescribing in the United Kingdom, with the majority of prescriptions for non-cancer pain. Morphine was the most frequently prescribed, but the utilization of oxycodone, buprenorphine and fentanyl increased markedly over time.
What’s already known about this topic? —
Studies in the United States regularly report increased opioid utilization and associated increased dose-related risks of dependence, misuse and mortality, but little is known in the United Kingdom.
What does this study add? —
Similar to the United States and some European countries, this study found an enormous increase in strong opioid prescribing in UK primary care, most commonly for non-cancer indications.
Although most patients were prescribed low doses, the utilization of newer opioids increased, although the daily morphine equivalent dose remained the same.
Further analysis of large patient databases will determine whether the harms associated with opioids are similar to those reported in the North America.
1. Introduction
Opioid analgesics have long been used as the gold standard to treat severe pain, most notably for acute pain and in palliative care. The use of opioids in chronic non-cancer pain (CNCP) has been much more controversial. Research from the United States (Zerzan et al., 2006; Sullivan et al., 2008), Australia (Leong et al., 2009), Canada (Fischer et al., 2011) and some European countries (Fredheim et al., 2010) have shown a significant increase in opioid utilization in the past decade, predominantly for patients with CNCP (Caudill-Slosberg et al., 2004; Gilson et al., 2004).
It is estimated that 90–95% of long-term opioid therapy is prescribed for non-cancer pain conditions and approximately 3% of the US population with non-cancer pain used opioids regularly for a month or more per year (Sullivan and Ferrell, 2005). However, there is a lack of robust evidence supporting the long-term opioid use in CNCP (Chapman et al., 2010), as the majority of the randomized controlled trials are of short duration (Chou et al., 2003, 2009; Noble et al., 2010). In addition, population-based research on the extent and characteristics of exposure to long-term opioids are also limited (Sullivan et al., 2008; Trescot et al., 2008).
A variety of opioids are available on the market with different clinical potencies, which can be roughly estimated by an equianalgesic ratio table (Gordon et al., 1999). The World Health Organization (WHO) considers a country’s opioid consumption as an indicator of progress in pain relief, especially for cancer pain (Scholten et al., 2007). Single- or cross-nation studies have reported both under- (Ponizovsky et al., 2012) and over- (Clausen, 1997) opioid utilization. Although increasing opioid prescribing for non-cancer patients may imply better attention to managing unresolved pain (Portenoy, 2004), widespread use of long-term opioids in CNCP has raised safety concerns. Recent studies suggest that long-term opioid use is associated with more frequent emergency department attendances and an increased incidence of side effects (Okie, 2010) and risk of opioid diversion and abuse, overdose and deaths (Gilson et al., 2007).
For patients receiving long-term opioid therapy for CNCP, opioid-related overdose death was found to be associated with higher prescribed doses (Bohnert et al., 2011). Specifically, the risk of drug-related adverse events was higher among patients prescribed doses greater than morphine 50 mg/day. Compared with patients receiving morphine 1–20 mg/day, patients receiving 50–99 mg/day or above 100 mg/day had a 3.7- and 8.9-fold increase in overdose risk, respectively (Dunn et al., 2010).
The aggregated dispensing data reported by the UK National Health Service (NHS) Information Centre suggests a large increase in opioid prescribing in the past decade (National Health Service. National Treatment Agency for Substance Misuse, 2011). It is likely that this increase is predominantly associated with CNCP. However, there is limited information on opioid prescribing patterns in CNCP and cancer pain in the United Kingdom. This study aimed to describe the trends of the most commonly prescribed strong opioids (buprenorphine, fentanyl, morphine and oxycodone) in a UK primary care setting from 2000 to 2010 stratified by cancer and non-cancer groups.
2. Methods
2.1 Study design and data source
This retrospective, cross-sectional study was conducted from 2000 to 2010 using the Clinical Practice Research Datalink (CPRD) after being granted approval from the Independent Scientific Advisory Committee of the Medicines and Healthcare Products Regulatory Agency for Database Research. CPRD is a longitudinal computerized database containing 5.2 million active patients’ anonymous medical records collected from approximately 636 primary care practices in the United Kingdom (Williams et al., 2012).
Prescriptions for four strong opioid (morphine and oxycodone, buprenorphine and fentanyl) issued during 2000–2010 were identified from CPRD by specific drug-related product codes. The four strong opioids are commonly prescribed for chronic pain management compared with other strong opioids (hydromorphone, meptazinol, pethidine) available in the United Kingdom. Injection and suppository prescriptions (3.1%) were excluded from this study. In addition, prescriptions for buprenorphine sublingual tablets 2 and 8 mg, which are almost exclusively used for managing opioid dependence instead of pain, were not included either. All selected opioid prescriptions were linked to the individual patient’s data file to extract demographic and diagnosis information. Adult users of strong opioids (aged 18–107 years old) with identifiable gender were included in this study. Prescriptions for the included patients were recorded for analysis up to 107 years old (the oldest age of patients registered in the CPRD database). The included strong opioid users were further stratified into cancer and non-cancer groups using specific medical codes for related cancer diagnosis.
Each prescription record contains information of item name and strength, prescription date, quantity and numerical daily dose (NDD). The NDD is a built-in information in CPRD that is transformed from the text records of administrative instructions. Less than 0.2% of prescriptions with quantity missing or extreme values (i.e., greater than two times of the 99th percentile value for quantity or day supply) were excluded from the analysis. The NDDs for prescriptions recorded as ‘as directed’ or ‘as required’ were identified as missing values (35.7%) and were replaced by the recommended number of daily dose from the British National Formulary according to an expert’s opinion.
2.2 Utilization measures
Utilization measures for the four opioids included annual number of prescriptions, number of patients, defined daily dose (DDD) for each opioid and oral morphine equivalent (OMEQ) dose per patient per day, and these measures were calculated in repeat cross-sectional estimates for each year and further stratified into cancer and non-cancer groups.
2.3 Number of prescriptions and users
The number of prescriptions and the number of prescriptions per patient in cancer and non-cancer groups were calculated annually. The number of patients prescribed strong opioid was repeatedly calculated by each calendar year, and patient and corresponding strong opioid prescriptions were categorized as either ‘cancer’ group after the first cancer diagnosis recorded; otherwise, they were included in the ‘non-cancer’ group. Patients’ demographic data (age and gender) were also recorded. Age was calculated based upon the date of the first prescription included in the study and stratified into five groups (i.e., ≤40, 41–50, 51–65, 66–80 and >80 years old).
2.4 Defined daily dose
The quantity of each prescription was multiplied by the strength (in milligrams) of the prescription to calculate the amount of each prescription. For transdermal buprenorphine and fentanyl formulations, the strength per hour and the duration of delivery rate of the formulation were considered in the dose calculation.
The annual total prescribed dose of each opioid entity was divided by the DDD (the daily average maintenance for a 70-kg male patient) of the particular opioid (Zerzan et al., 2006; Hamunen et al., 2008), as defined by the WHO Collaborating Centre for Drug Statistics based upon the Anatomical Therapeutic Chemical classification system (WHO Collaborating Centre for Drug Statistics Methodology, 2013). The result was then divided by the total number of patients registered in the CPRD for the year, and then multiplied by 1000 and further divided by 365 to derive the annual number of DDDs per 1000 inhabitants per day, which was used as an indicator for the prevalence of strong opioid utilization.
2.5 Oral morphine equivalent dose
The dose for each prescription was multiplied by the equianalgesic ratio of the opioid (Mercadante et al., 2007; Sullivan et al., 2008; Svendsen et al., 2011) to derive the OMEQ dose. The number of ‘days of supply’ for each prescription was calculated by dividing the quantity by the NDD. Total days of supply of prescriptions for each patient per calendar year were calculated and the overlapping days of supply between prescriptions within a year were subtracted. Annual OMEQ dose per day of supply was calculated by dividing the total OMEQ dose by the total days of supply for each patient in a calendar year. The annual number of users was further stratified by the four daily OMEQ dose ranks, including ≤50 (low dose rank), 51–100, 101–200 and >200 mg/day (higher dose ranks). The contribution of each of the four opioids to each OMEQ dose rank was calculated.
2.6 Data analysis
Descriptive statistics were used to report outcome variables for each year, including number of strong opioids users, number of opioid prescriptions, number of prescriptions per patient and opioid dose per day. Linear trend analysis was conducted on annual outcome measures and the percentage change between 2000 and 2010 data for each variable was also reported. Sensitivity analyses were conducted to explore the impacts of NDD missing data management on the dose calculation. All analyses were conducted using Stata 12 (Stata Corp LP, College Station, TX, USA, 2011).
3. Results
3.1 Number of strong opioid users
In total, 2,672,022 prescriptions of the four strong opioid analgesics were prescribed for 178,692 users (59.9% female) during the 11-year study period (Table 1). The mean age of strong opioid users was 67.1 ± 17.0 years (mode: 77; range: 18–107 years). Of the five age ranks, there was a higher proportion of patients aged 66–80 years old (33.6%), followed by aged more than 80 (24.4%) and 51–65 years old (23.8%). The number of strong opioid users each year increased over time from 9479 to 53,666 (466.2% increase) during the study period, and this represents 1.8–9.2 per thousand patients that were registered in CPRD.
Table 1.
Characteristics of strong opioid users included during study period
| Total opioid users | Non-cancer group | Cancer group | |
|---|---|---|---|
| Number of patients | 178,692 (100%) | 149,896 (83.9%) | 28,796 (16.1%) |
| Gender | |||
| Male | 71,638 (40.1%) | 56,392 (37.6%) | 15,246 (52.9%) |
| Female | 107,054 (59.9) | 93,504 (62.4) | 13,550 (47.1%) |
| Age (years) | |||
| Mean ± SD (range) | 67.1 ± 17.0 (18–107) | 66.6 ± 17.6 (18–107) | 69.7 ± 12.9 (18–106) |
| Mode | 77 | 80 | 74 |
| Rank of age | |||
| ≤40 | 14,928 (8.4%) | 14,318 (9.6%) | 610 (2.1%) |
| 41–50 | 17,625 (9.9%) | 15,937 (10.6%) | 1,688 (5.9%) |
| 51–65 | 42,521 (23.8%) | 34,746 (23.2%) | 7,775 (27.0%) |
| 66–80 | 60,091 (33.6%) | 47,464 (31.7%) | 12,627 (43.9%) |
| >80 | 43,527 (24.4%) | 37,431 (25.0%) | 6,096 (21.2%) |
SD, standard deviation.
Of all strong opioid users, 28,796 (16.1%) users had cancer diagnoses recorded during the study period and any subsequent prescriptions were categorized as the ‘cancer group’, and the remaining 149,896 (83.9%) patients without a cancer diagnosis were categorized as the ‘non-cancer group’. There was a higher proportion of female patients in the non-cancer group (62.4%) than the cancer group (47.1%). The total increase in the annual number of female strong opioid users (537.7%) was higher than that of male users (372.6%), and similarly, a higher increase of female users was found in both non-cancer (575.3% vs. 414.3%) and cancer groups (300.0% vs. 216.7%).
The mean age of strong opioid users was slightly lower in the non-cancer group (66.6 ± 17.6; mode: 80; range: 18–107 years) than the cancer group (69.7 ± 12.9; mode: 74; range: 18–106 years). In the non-cancer group, there was a slightly larger proportional increase in the number of patients in the youngest (<40 years) and oldest (>80 years) compared with other age groups over the study period.
3.2 Number of prescriptions
Prescriptions for the four strong opioids (n = 2,672,022) accounted for 94.7% of the total number of strong opioid prescriptions identified during study period, and a majority (n = 2,347,282; 87.8%) of those strong opioid prescriptions were issued without or prior to a cancer diagnosis record and categorized as the non-cancer group; only 12.1% prescriptions (n = 324,740) were issued with or following to a cancer diagnosis.
For all patients, the total number of prescriptions per patient increased overtime from 5.7 in 2000 to 9.4 in 2010 (64.7%). The mean number of prescriptions issued per patient per year was slightly higher in the non-cancer group (from 6.0 in 2000 to 9.5 in 2010) than in the cancer group (from 4.6 to 8.8) during the study period. However, the increase in the annual number of prescriptions per patient in non-cancer group (59.6 %) was lower than in the cancer group (91.2%) (Fig. 1).
Figure 1.
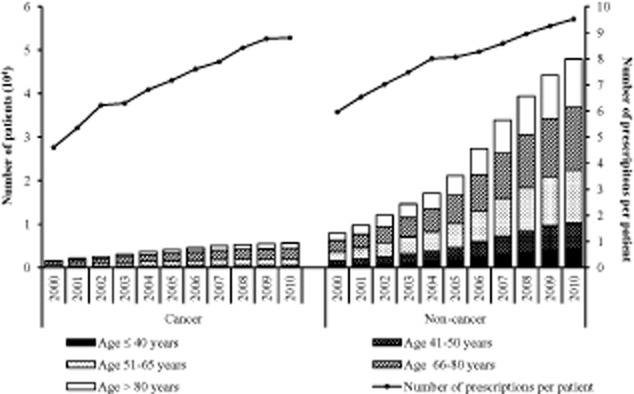
Number of strong opioid prescriptions and users in non-cancer and cancer groups.
Of the four strong opioids, morphine was the most frequently prescribed opioid, with the highest proportion in total number of prescriptions in both the non-cancer (47.3%) and the cancer groups (61.4%), followed by buprenorphine (18.6% vs. 4.7%), fentanyl (18.4% vs. 17.0%) and oxycodone (15.5% vs. 16.7%) (Fig. 2). However, the greatest increase in annual number of prescriptions was for oxycodone in both the non-cancer (11,265.5%, from 764 to 86,833) and cancer groups (8939.5%, from 124 to 11,209), followed by buprenorphine (1650.6% vs. 4865.4%), fentanyl (1283.5% vs.765.0%) and morphine (422.3% vs. 324.6%).
Figure 2.
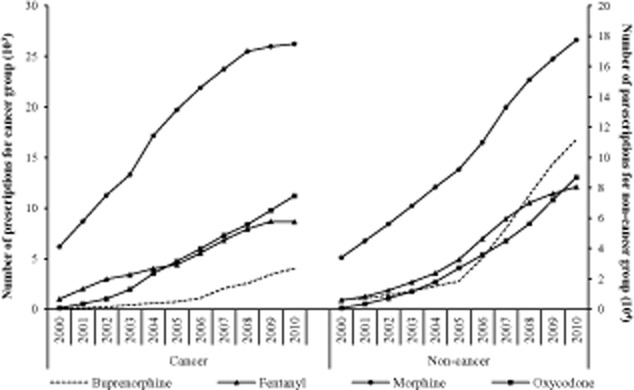
Number of prescriptions for each strong opioid in non-cancer and cancer groups.
3.3 Defined daily dose
Of all four strong opioids, morphine had the highest mean annual DDD (0.85 ± 0.33 per 1000 inhabitants per day) during the study period, followed by fentanyl (0.52 ± 0.32), oxycodone (0.28 ± 0.21) and buprenorphine (0.24 ± 0.16). The mean annual DDD (per 1000 inhabitants per day) was higher in the non-cancer group than in the cancer group for all four strong opioids, that is, morphine (0.73 ± 0.28 vs. 0.12 ± 0.04), fentanyl (0.46 ± 0.29 vs. 0.06 ± 0.24), oxycodone (0.24 ± 0.19 vs. 0.038 ± 0.028) and buprenorphine (0.23 ± 0.15 vs. 0.008 ± 0.006).
The increase in annual DDD (per 1000 inhabitants per day) in both non-cancer and cancer groups was similar to the number of prescriptions of the four strong opioids, in which oxycodone had the highest compared to buprenorphine, fentanyl and morphine (Fig. 3).
Figure 3.
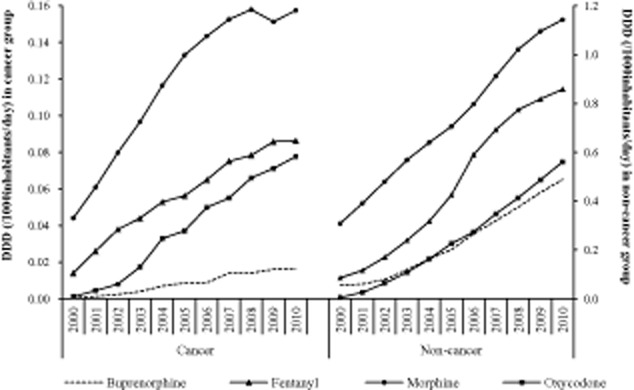
Number of defined daily dose (DDD)/1000 inhabitants/day for each strong opioid in non-cancer and cancer groups.
3.4 Annual days of supply
The mean annual days of supply per patient during the study period were 124.9 ± 121.6 days, and this was longer for patients in the non-cancer group (130.6 ± 124.2 days) than in the cancer group (88.9 ± 95.8 days). There was an increase in the mean annual days of supply per patient over the study period (56.9%), but this was higher in the cancer group (82.1%; 58.3–106.1 days) than in the non-cancer group (50.5%; 98.5–148.4 days) (Fig 4).
Figure 4.
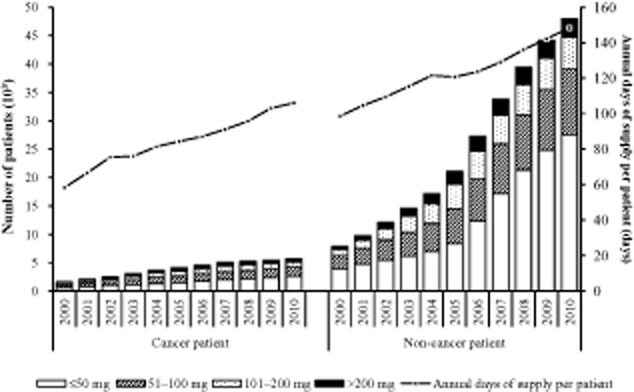
Number of patients stratified by four dose ranks for annual oral morphine equivalent (OMEQ) dose per patient per day.
3.5 Oral morphine equivalent dose
The mean OMEQ for the total patient population over the study period was 88.9 ± 8.7 mg/day and was slightly higher for patients with cancer diagnoses (105.9 ± 6.1 mg/day) than with non-cancer diagnoses (86.0 ± 8.7 mg/day) (Fig. 5).
Figure 5.
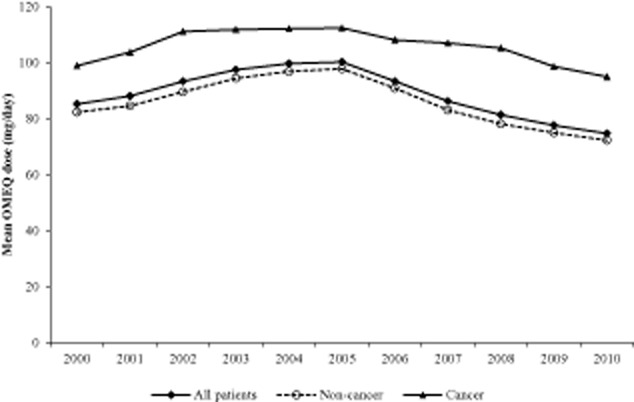
Annual oral morphine equivalent (OMEQ) dose per patient per day in all patients, non-cancer and cancer groups.
Of the four dose ranks of annual OMEQ dose per patient day (Fig. 4), there was a higher proportion of patients in the low dose rank (≤50 mg/day) in both the non-cancer (50.3%) and the cancer (39.9%) groups, followed by the dose ranks of 51–100 mg/day (26.2% vs. 28.7%), 101–200 mg/day (15.1% vs. 19.2%) and >200 mg/day (8.25% vs. 12.1%). The greatest increase in the number of patients over time was in the lowest OMEQ dose rank for both the non-cancer (606.2%) and the cancer (309.1%) groups compared with the increases in other dose ranks, that is, 51–100 mg/day (397.3% vs. 217.0%), 101–200 mg/day (430.6% vs. 206.1%) and >200 mg/day (447.8% vs. 252.5%) during the 11-year study period (Fig. 4).
The highest proportion of dose in the low OMEQ rank (≤50 mg/day) was contributed by morphine (45.2% vs. 72.1%), followed by buprenorphine (37.1% vs. 12.5%), oxycodone (11.1% vs. 10.4%) and fentanyl (6.6% vs. 4.9%) in non-cancer and cancer groups, respectively. However, there was a decreasing trend in the proportion of OMEQ dose contributed by morphine over time across the four dose ranks (Fig. 6). In contrast, the proportion of OMEQ dose contributed by oxycodone increased over time in all dose ranks.
Figure 6.
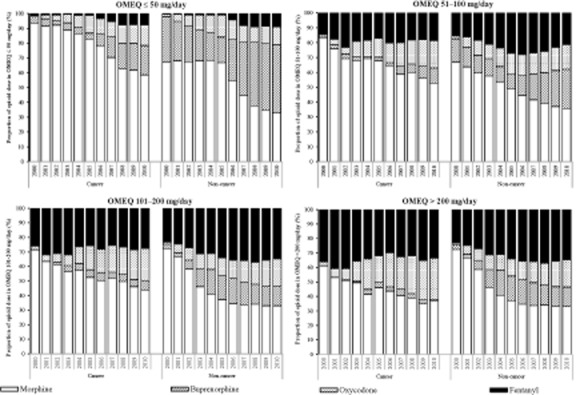
Proportion of opioids contributing to each rank of oral morphine equivalent (OMEQ) calculation between cancer and non-cancer patients.
In both study groups, the proportion of OMEQ contributed by buprenorphine also increased over time in all dose ranks, but predominantly this increase was in the low OMEQ rank (≤50 mg/day) from 30.9% to 45.9% in the non-cancer group versus 3.9% to 19.8% in the cancer group. The proportion of OMEQ contributed by fentanyl increased over time in the low OMEQ rank (0.5–8.9% in the non-cancer vs. 1.5–7.9% in cancer groups), but the proportions remained consistent in other dose ranks in both study groups. These trend changes in the proportions of OMEQ dose contributed by different opioids over time were consistent in both the cancer and the non-cancer patient groups.
4. Discussion
Consistent with opioid utilization in other European countries (Hamunen et al., 2009; Fredheim et al., 2010), this cross-sectional study found a huge increase in strong opioid prescribing in a UK primary care setting from 2000 to 2010, and the majority was prescribed for non-cancer patients. A greater increase in prescriptions than the number of patients exposed to strong opioids, and increasing days of supply were also consistently found across the 11-year period.
Compared with recent national dispensing data (National Health Service. The Information Centre for Health and Social Care, 2013), this study included about 10% of strong opioid prescriptions in the United Kingdom, which coincides with approximately 8% of the UK population registered with CPRD (Lawrenson et al., 1999). Despite adopting an arbitrary definition to categorize patients with cancer or non-cancer diagnosis, this may have possibly under- or overestimated the strong opioid prescriptions for non-cancer (87.8%) and cancer pain (12.1%). However, the results are comparable with a Danish study that found 9.5% of opioid prescriptions were issued for cancer pain by general practitioners over a 12-month period (Clausen, 1997). The concordance of recording cancer diagnoses in CPRD with national cancer registries is relatively high (83.3%) (Boggon et al., 2013) and the most common primary tumour sites in 29,825 patients registered with CPRD who died between 2000 and 2008 were lung (34.2%), colorectal (19.9%), female breast (21.6%) and prostate (19.1%) (Higginson and Gao, 2012).
The predominant strong opioid users were between 66 and 80 years old in both the non-cancer and the cancer patient groups. This is perhaps unsurprising as older people report more pain conditions and are more likely to be prescribed with opioids than younger population (Parsells Kelly et al., 2008; Fredheim et al., 2010). However, the increase in prescribing for the youngest age group (<40 years) and consequent potential increased exposure is concerning given the increased understanding of harms associated with long-term opioid use.
Previous research has also suggested that female patients are more likely to report a range of chronic pain conditions (Unruh, 1996), and more frequently report severe pain, longer lasting pain and anatomically diffuse pain than male patients (Hurley and Adams, 2008). This study also demonstrated clear variation in gender; the majority of non-cancer strong opioid users were female (62.4%) but the proportions of female and male were similar in the cancer group. This finding is consistent with the results of a Norwegian study from 2004 to 2007 that evaluated opioid dispensing data with reimbursement codes for identifying cancer that reported a higher proportion of female patients (57%) in a non-cancer group and a similar proportion between female (49%) and male patients in the cancer group (Fredheim et al., 2010).
This study reported both DDD and OMEQ dose to measure the utilization of strong opioids. DDD per 1000 inhabitants per day reflects the proportion of the population in primary care, on average, that receive a particular drug daily, and this measure allows cross-nation comparison of drug exposure (WHO International Working Group for Drug Statistics Methodology. WHO Collaborating Centre for Drug Statistics Methodology. WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services, 2003). Of the four strong opioids, this study found a higher proportion of patients exposed to morphine, but the increase of proportion was greater for oxycodone, buprenorphine and fentanyl in both non-cancer and cancer groups over time. A similar pattern was also observed in an opioid utilization study comparing across five Nordic countries (Denmark, Norway, Sweden, Finland and Iceland) (Hamunen et al., 2009).
Hamunen et al. (2009) evaluated the trends of opioid consumption presented as DDDs/1000 inhabitants/day for patients (with non-cancer or cancer conditions) in both hospital and primary care settings from 2002 to 2006 using national reimbursement databases of the five Nordic countries, and found morphine consumption was stable or slightly decreased in all countries. However, fentanyl consumption increased in Denmark, Finland and Sweden, oxycodone consumption dramatically increased in all countries except Iceland, and buprenorphine consumption also increased in all countries (Hamunen et al., 2009). Increasingly, oxycodone is considered the first choice opioid analgesic for opioid-naive patients by both hospital physicians and general practitioners (Poulsen et al., 2013).
Although DDD is considered to be the best available tool of measuring drug utilization in cross-nation comparison studies, it does not consider equianalgesic doses of different opioid (Hamunen et al., 2008). Therefore, this study also considered OMEQ to measure opioid use and found that the majority of patients in both non-cancer and cancer groups were prescribed a low daily OMEQ dose, and the number and proportion of patients in the low dose rank increased over time. In the non-cancer group, the proportion of dose calculation contributed by buprenorphine in low OMEQ dose rank dramatically increased after 2005, and this trend coincides with the launch of 7-day patch formulation in 2005.
With the increase in both numbers of strong opioid users and prescriptions, the numbers of patients who were prescribed with strong opioids in the higher dose ranks also increased, which raises drug safety concerns. Other research have suggested that patients receiving higher dose regimens are more likely to deviate from medically prescribed use (e.g., increasing dose above the prescribed levels, using opioids that were not prescribed or using other substances that influence overdose risks) (Dunn et al., 2010) and several studies conducted in the United States have also demonstrated that higher opioid doses were associated with increased risk of opioid overdose death (Dunn et al., 2010; Bohnert et al., 2011).
Although morphine was the main contributor to the higher OMEQ dose ranks (51–100, 101–200 and >200 mg/day), the proportion decreased throughout the study period. In contrast, the proportions contributed by oxycodone and buprenorphine increased in higher dose ranks over time. The increasing contribution of oxycodone to higher opioid doses has also been found in a nested case–control study conducted in Canada (Gomes et al., 2011), which reported a direct association between opioid dose and opioid-related mortality, in which high (200–400 mg/day) and very high (>400 mg/day) daily doses were associated with double the all-cause mortality but five to six times of the opioid-related mortality rate. Oxycodone and, to a lesser extent, fentanyl were the main contributors to doses exceeding 200 mg morphine equivalent per day.
The study took a cross-sectional trend design to evaluate the trends of strong opioid utilization over 11 years using a representative and well-recorded primary care dataset (Walley and Mantgani, 1997), which has been previously used for research on analgesics and pain (Hall et al., 2006; Gao et al., 2011) to avoid recall bias, and included all users of the four strong opioids to avoid selection bias. Sensitivity analyses exploring the management of missing data found no significant influence of the imputation procedure on dose calculation. However, as this study only included the prescribing data of strong opioids, it may overestimate the actual consumption in primary care. However, the publicly available summary of primary care dispensing data for all of England and Wales showed a trend similar to this study (National Health Service. The Information Centre for Health and Social Care, 2013).
In contrast to the traditional cohort study approach, this study took a simplistic definition to stratify the cancer and non-cancer groups after a first cancer diagnosis was recorded for a patient, thus could possibly underestimate the utilization of strong opioids for non-cancer conditions. Without longitudinal follow-up for individual patient’s diseases and their persistence of strong opioid utilization, it was not possible to differentiate whether the strong opioids were prescribed for acute or chronic pain conditions, and whether the prescribed opioids were taken by patients. Likewise, we were not able to judge whether the change of utilization trends indicate improvement in pain management as clinical outcomes were not evaluated.
5. Conclusions
To our knowledge, this is the first large-scale observational study describing the trends in strong opioid prescribing within UK primary care settings over an 11-year study period that stratified patients into cancer and non-cancer groups. Although the definitions used to stratify the non-cancer and cancer groups may result in overestimation of strong opioids prescribed for cancer pain in primary care, this study found an escalating of strong opioid prescribing in the UK primary care setting between 2000 and 2010, predominately prescribed for non-cancer patients. Similar patterns of opioid utilization have been reported across European countries, and the decrease of morphine seems to be complemented by an increase of oxycodone, fentanyl and buprenorphine.
To evaluate the appropriateness of opioid utilization (over- or under-prescribing), further well-designed longitudinal studies are needed to evaluate the effectiveness and safety of long-term opioid therapy pain management, particularly the dose-related risk and benefit profiles in elderly patients with non-cancer pain conditions. Being equally effective and cheaper than other opioid analgesics, morphine has long been recommended as the gold standard for pain therapy (Bekkering et al., 2011). However, with increasing availability of opioids in novel formulations (such as transdermal fentanyl and buprenorphine) and better efficacy or safety profiles (Bekkering et al., 2011), clinical guidance on opioid analgesics need to consider not only robust clinical and economic evidence but also other factors (such as convenience of dosing, doctor/patient preferences, anticipated adverse effects) in determining the choice of opioid.
Author contributions
L.-C.C. and R.D.K. both initiated and developed the research questions and study design. L.-C.C. was in charge of accessing the research data and overseeing the whole research project. C.S.Z. conducted data management and analysis, and led on drafting the manuscript. All of the authors contributed to the interpretation of the data, critically revised the manuscript and approved the final version submitted for publication.
References
- Bekkering GE, Soares-Weiser K, Reid K, Kessels AG, Dahan A, Treede RD, Kleijnen J. Can morphine still be considered to be the standard for treating chronic pain? A systematic review including pair-wise and network meta-analyses. Curr Med Res Opin. 2011;27:1477–1491. doi: 10.1185/03007995.2011.586332. [DOI] [PubMed] [Google Scholar]
- Boggon R, van Staa TP, Chapman M, Gallagher AM, Hammad TA, Richards MA. Cancer recording and mortality in the General Practice Research Database and linked cancer registries. Pharmacoepidemiol Drug Saf. 2013;22:168–175. doi: 10.1002/pds.3374. [DOI] [PubMed] [Google Scholar]
- Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 versus 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Chapman CR, Lipschitz DL, Angst MS, Chou R, Denisco RC, Donaldson GW, Fine PG, Foley KM, Gallagher RM, Gilson AM, Haddox JD, Horn SD, Inturrisi CE, Jick SS, Lipman AG, Loeser JD, Noble M, Porter L, Rowbotham MC, Schoelles KM, Turk DC, Volinn E, Von Korff MR, Webster LR, Weisner CM. Opioid pharmacotherapy for chronic non-cancer pain in the United States: A research guideline for developing an evidence-base. J Pain. 2010;11:807–829. doi: 10.1016/j.jpain.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Chou R, Clark E, Helfand M. Comparative efficacy and safety of long-acting oral opioids for chronic non-cancer pain: A systematic review. J Pain Symptom Manage. 2003;26:1026–1048. doi: 10.1016/j.jpainsymman.2003.03.003. [DOI] [PubMed] [Google Scholar]
- Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: Findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:147–159. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Clausen TG. International opioid consumption. Acta Anaesth Scand. 1997;41:162–165. doi: 10.1111/j.1399-6576.1997.tb04632.x. [DOI] [PubMed] [Google Scholar]
- Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von KM. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Jones W, Krahn M, Rehm J. Differences and over-time changes in levels of prescription opioid analgesic dispensing from retail pharmacies in Canada, 2005–2010. Pharmacoepidemiol Drug Saf. 2011;20:1269–1277. doi: 10.1002/pds.2190. [DOI] [PubMed] [Google Scholar]
- Fredheim OMS, Skurtveit S, Breivik H, Borchgrevink PC. Increasing use of opioids from 2004 to 2007: Pharmacoepidemiological data from a complete national prescription database in Norway. Eur J Pain. 2010;14:289–294. doi: 10.1016/j.ejpain.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Gao W, Gulliford M, Higginson IJ. Prescription patterns of analgesics in the last 3 months of life: A retrospective analysis of 10 202 lung cancer patients. Br J Cancer. 2011;104:1704–1710. doi: 10.1038/bjc.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson AM, Maurer MA, Joranson DE. State medical board members’ beliefs about pain, addiction, and diversion and abuse: A changing regulatory environment. J Pain. 2007;8:682–691. doi: 10.1016/j.jpain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997–2002. J Pain Symptom Manage. 2004;28:176–188. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- Gordon DB, Stevenson KK, Griffie J, Muchka S, Rapp C, Ford-Roberts K. Opioid equianalgesic calculations. J Palliat Med. 1999;2:209–218. doi: 10.1089/jpm.1999.2.209. [DOI] [PubMed] [Google Scholar]
- Hall GC, Carroll D, Parry D, McQuay HJ. Epidemiology and treatment of neuropathic pain: The UK primary care perspective. Pain. 2006;122:156–162. doi: 10.1016/j.pain.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Hamunen K, Laitinen-Parkkonen P, Paakkari P, Breivik H, Gordh T, Jensen NH, Kalso E. What do different databases tell about the use of opioids in seven European countries in 2002? Eur J Pain. 2008;12:705–715. doi: 10.1016/j.ejpain.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Hamunen K, Paakkari P, Kalso E. Trends in opioid consumption in the Nordic countries 2002–2006. Eur J Pain. 2009;13:954–962. doi: 10.1016/j.ejpain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Higginson IJ, Gao W. Opioid prescribing for cancer pain during the last 3 months of life: Associated factors and 9-year trends in a nationwide united kingdom cohort study. J Clin Oncol. 2012;30:4373–4379. doi: 10.1200/JCO.2012.42.0919. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Adams MCB. Sex, gender, and pain: An overview of a complex field. Anesth Analg. 2008;107:309–317. doi: 10.1213/01.ane.0b013e31816ba437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson R, Williams T, Farmer R. Clinical information for research; the use of general practice databases. J Public Health. 1999;21:299–304. doi: 10.1093/pubmed/21.3.299. [DOI] [PubMed] [Google Scholar]
- Leong M, Murnion B, Haber PS. Examination of opioid prescribing in Australia from 1992 to 2007. Intern Med J. 2009;39:676–681. doi: 10.1111/j.1445-5994.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Villari P, Ferrera P, Casuccio A, Mangione S, Intravaia G. Transmucosal fentanyl vs intravenous morphine in doses proportional to basal opioid regimen for episodic-breakthrough pain. Br J Cancer. 2007;96:1828–1833. doi: 10.1038/sj.bjc.6603811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1) doi: 10.1002/14651858.CD006605.pub2. CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138:507–513. doi: 10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Ponizovsky AM, Pchelintsev MV, Marom E, Zvartau EE. Differences in the consumption rates and regulatory barriers to the accessibility of strong opioid analgesics in Israel and St. Petersburg. Eur J Clin Pharmacol. 2012;68:89–95. doi: 10.1007/s00228-011-1099-z. [DOI] [PubMed] [Google Scholar]
- Portenoy RK. Appropriate use of opioids for persistent non-cancer pain. Lancet. 2004;364:739–740. doi: 10.1016/S0140-6736(04)16951-1. [DOI] [PubMed] [Google Scholar]
- Poulsen KK, Andersen SE, Moreno SI, Glintborg D, Thirstrup S, Aagaard L. General practitioners’ and hospital physicians’ preference for morphine or oxycodone as first-time choice for a strong opioid: A national register-based study. Basic Clin Pharmacol Toxicol. 2013;112:110–115. doi: 10.1111/j.1742-7843.2012.00927.x. [DOI] [PubMed] [Google Scholar]
- Scholten W, Nygren-Krug H, Zucker HA. The World Health Organization paves the way for action to free people from the shackles of pain. Anesth Analg. 2007;105:1–4. doi: 10.1213/01.ane.0000267542.72315.34. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Ferrell B. Ethical challenges in the management of chronic nonmalignant pain: Negotiating through the cloud of doubt. J Pain. 2005;6:2–9. doi: 10.1016/j.jpain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Fan M-Y, DeVries A, Braden JB, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in Commercial and Medicaid insurance plans: The TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen K, Borchgrevink PC, Fredheim O, Hamunen K, Mellbye A, Dale O. Choosing the unit of measurement counts: The use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliati Med. 2011;25:725–732. doi: 10.1177/0269216311398300. [DOI] [PubMed] [Google Scholar]
- Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician. 2008;11:S181–S200. [PubMed] [Google Scholar]
- Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Walley T, Mantgani A. The UK general practice research database. Lancet. 1997;350:1097–1099. doi: 10.1016/S0140-6736(97)04248-7. [DOI] [PubMed] [Google Scholar]
- WHO International Working Group for Drug Statistics Methodology. WHO Collaborating Centre for Drug Statistics Methodology. WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. 2003. Introduction to Drug Utilization Research.
- Williams T, Van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf. 2012;3:89–99. doi: 10.1177/2042098611435911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerzan JT, Morden NE, Soumerai S, Ross-Degnan D, Roughead E, Zhang F, Simoni-Wastila L, Sullivan SD. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Med Care. 2006;44:1005–1010. doi: 10.1097/01.mlr.0000228025.04535.25. [DOI] [PubMed] [Google Scholar]
Web references
- National Health Service. National Treatment Agency for Substance Misuse. 2011. Addiction to medicine: An investigation into the configuration and commissioning of treatment services to support those who develop problems with prescription-only or over-the-counter medicine. Retrieved from: http://www.nta.nhs.uk/uploads/addictiontomedicinesmay2011a.pdf (accessed on 25 February 2013)
- National Health Service. The Information Centre for Health and Social Care. 2013. Prescribing cost analysis England. Retrieved from: http://www.ic.nhs.uk/statistics-and-data-collections/primary-care/prescriptions (accessed on 25 February 2013)
- WHO Collaborating Centre for Drug Statistics Methodology. 2013. Guidelines for ATC classification and DDD assignment. Retrieved from: http://www.whocc.no/atc_ddd_index/ (accessed on 25 February 2013)


