Abstract
Background
The reliable prediction of hepatocellular carcinoma (HCC) recurrence patterns potentially allows for the prioritization of patients for liver resection (LR) or transplantation.
Objectives
The aim of this study was to analyse clinicopathological factors and preoperative Milan criteria (MC) status in predicting patterns of HCC recurrence.
Methods
During 1992–2012, 320 patients undergoing LR for HCC were categorized preoperatively as being within or beyond the MC, as were recurrences.
Results
After a median follow-up of 47 months, 183 patients developed recurrence, giving a 5-year cumulative incidence of recurrence of 62.5%. Patients with preoperative disease within the MC had better survival outcomes than those with preoperative disease beyond the MC (median survival: 102 months versus 45 months; P < 0.001). Overall, 31% of patients had preoperative disease within the MC and 69% had preoperative disease beyond the MC. Estimated rates of recurrence-free survival at 5 years were 61.8% for all patients and 53.8% for patients with initial beyond-MC status. Independent factors for recurrence beyond-MC status included preoperative disease beyond the MC, the presence of microsatellite or multiple tumours and lymphovascular invasion (all: P < 0.001). A clinical risk score was used to predict survival and the likelihood of recurrence beyond the MC; patients with scores of 0, 1, 2 and 3 had 5- year incidence of recurring beyond-MC of 9.0%, 29.5%, 48.8% and 75.4%, respectively (P < 0.0001).
Conclusions
Regardless of initial MC status, at 5 years the majority of patients remained disease-free or experienced recurrence within the MC after LR, and thus were potentially eligible for salvage transplantation (ST). Incorporating clinicopathological parameters into the MC allows for better risk stratification, which improves the selection of patients for ST and identifies patients in need of closer surveillance.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and one of the leading causes of cancer-related death.1 Its incidence in the USA has tripled over the last 20 years and is related to the dramatic increases in hepatitis C virus-related and alcoholic liver disease.2 Surgery, either liver resection (LR) or orthotopic liver transplantation (OLT), remains the standard of care in the curative treatment of HCC.3 Liver resection is the treatment of choice for patients with HCC without cirrhosis and provides excellent early and longterm results.4 The care of HCC patients was revolutionized after Mazzaferro et al. demonstrated in 1996 that patients with up to three HCC nodules, each of <3 cm in size, or one tumour of <5 cm, without vascular invasion or extrahepatic spread [factors that became known as the ‘Milan criteria’ (MC)] experienced a 5-year overall survival (OS) rate comparable with that in patients with end-stage liver cirrhosis undergoing transplant without cancer (75%).5 The MC is the current benchmark for selecting HCC patients for OLT: it has been validated in many studies including a recent meta-analysis, and is used by the United Network for Organ Sharing (UNOS) and most transplant centres.5–8
In theory, OLT represents an ideal choice for patients with cirrhosis and HCC because it treats the underlying liver disease as well as the tumour and is associated with fewer recurrences than resection. However, long waiting lists due to the lack of suitable donor organs, the potential for tumour progression beyond the MC while awaiting transplantation and problems related to lifelong immunosuppression weaken its potential benefits.9,10 Indeed, when intention-to-treat analysis is used, resection and OLT appear to have comparable longterm survival rates.7,9,11,12
The strategy of utilizing LR as the initial treatment option in transplant-eligible HCC patients and employing salvage transplantation (ST) for HCC recurrences is both reasonable and attractive. Recent studies have shown promising results from combining these two primary therapeutic modalities, utilizing resection as the first-line intervention and reserving transplantation listing (for ST) for patients who show favourable histopathological features following resection, or in the event of recurrence within the MC.11,13–18 Most of these studies have involved patients with tumours within the MC on a background of cirrhosis. At the same time, adverse histopathological features, such as vascular invasion, poor differentiation, high-grade disease and microsatellitosis, are known to increase as the tumour size and number increase beyond the MC.12,19,20 Additionally, recent reports have identified several independent clinicopathological risk factors for HCC recurrence, such as serum albumin level, indocyanine green retention rate at 15 min (ICG R15), anatomical resection, tumour number, vascular invasion, beyond MC status and prior transcatheter arterial chemoembolization (TACE).21,22
This study investigated the relationship between preoperative MC status and HCC recurrence in a surgical cohort comprising patients with advanced-stage tumours arising mostly in non-cirrhotic livers. It also investigated clinicopathological features predictive of patterns of recurrence which can enhance the ability of the MC to identify patients with unfavourable biology, who might be candidates for ST or might perhaps be better served by initial transplantation. Based on this analysis, a risk model was generated.
Materials and methods
Patient selection and preoperative assessment
Patients with HCC who underwent LR with curative intent and negative surgical margins at Memorial SloanKettering Cancer Center (MSKCC), between January 1992 and January 2012, were identified from a prospective database. Patients with fibrolamellar HCC or mixed hepatocellular–cholangiocarcinoma cancers were excluded. Similarly, patients who underwent ablation only without LR and patients who underwent LR at an outside facility were excluded. Medical records were reviewed for clinical and pathological variables including age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) score, comorbidities (such as liver cirrhosis, aetiology of liver disease, hepatitis status), laboratory results [e.g. serum albumin, bilirubin, platelet count, liver function, α-fetoprotein (AFP)], prior treatment (e.g. TACE, prior LR/ablation), preoperative tumour size and number, operative data (type of LR, blood loss/transfusion, operative time, additional procedures), pathological data (tumour size, microsatellites, margins, tumour differentiation, lymphovascular invasion), and recurrence and survival data. Approval for the study was obtained from the MSKCC institutional review board.
The preoperative management of patients was discussed at weekly multidisciplinary hepatopancreatobiliary (HPB) disease management conference which included HPB surgeons, medical oncologists, diagnostic and interventional radiologists, and gastroenterologists. Criteria for resectability included anatomically resectable disease with an adequate future liver remnant (FLR) reserve, the absence of distant metastases and the absence of main portal vein and inferior vena cava tumour thrombus. The preoperative assessment of patients included a complete history and physical examination, complete blood count and metabolic panel, liver function panel, coagulation profile and AFP level. Routine imaging consisted of a triphasic computed tomography (CT) or magnetic resonance imaging (MRI) of the liver and a CT of the chest as part of the metastatic workup. Volumetry by CT was performed in selected patients in whom concerns regarding FLR volume, based on the extent of the planned resection, existed. Patients were considered positive for hepatitis B virus if they had hepatitis B surface antigen or anti-hepatitis B core antibody, and considered positive for hepatitis C virus if they had anti-hepatitis C virus antigen.
Patients were assessed according to the MC at their initial presentation and at any recurrence. Patients were stratified by MC status based on preoperative imaging. Microsatellites were determined by histopathological examination; the presence of multiple tumours was based on preoperative cross-sectional imaging.
Surgical resection and histopathology
All resections were performed using a standard technique as reported previously.23 Intraoperative ultrasonography was routinely performed to detect additional tumours in the liver, to delineate the relationship of the tumour to major vascular structures, and to guide the plane of parenchymal transection. Resections were either anatomic or limited according to the severity of cirrhosis and the location(s) of the tumour(s). An intermittent Pringle manoeuvre was used selectively; especially in major resections. Major resections were defined using the International Hepatopancreatobiliary Association (IHPBA) Brisbane classification as consisting of three or more Couinaud liver segments.24 The diagnosis of HCC was confirmed pathologically in all patients. The histopathological features of the tumour(s), including number, size of the greatest lesion, lymphovascular invasion, satellite lesions, and presence of cirrhosis were recorded. Tumours were graded as being well, moderately or poorly differentiated.
Follow-up and recurrences
After the initial postoperative outpatient visit, the follow-up schedule consisted of office visits every 3–4 months in the first 2 years and every 6 months thereafter, during which physical examination, liver function tests, AFP levels and CT and/or MRI were routinely performed. Recurrences were either biopsy-proven or evidenced on radiology (according to the typical appearance on CT or MRI of new imaging findings that progressed in serial imaging). The date of recurrence was recorded as the date of any radiological study identifying new findings, and the type of recurrence was classified as being within or beyond the MC and recorded. Recurrence-free survival (RFS) and OS were calculated from the date of LR until tumour recurrence or patient death/last follow-up, and from the date of LR until patient death/last follow-up, respectively. Sites of initial disease recurrence were also recorded and liver-only disease was distinguished from extrahepatic recurrences.
Recurrences were treated using a variety of techniques, mainly re-resection and interventional radiology-guided radiofrequency ablation, microwave ablation, ethanol injection or transarterial embolization (TAE). Treatment decisions for recurrent disease were discussed during a multidisciplinary disease management team conference. Medically fit patients who developed recurrence were offered re-resection if the disease was resectable and in the absence of contraindications (i.e. an adequate liver remnant, absence of multifocal disease, absence of extrahepatic metastases, etc.). Other locoregional treatments, such as TAE and/or ablation, were offered to patients in whom resection was inappropriate but ablative therapy was technically feasible. Systemic therapy, such as treatment with sorafenib (Nexavar™; Bayer HealthCare Pharmaceuticals, Inc., Whippany, NJ, USA) or treatment on clinical trials, was generally offered to selected patients with extrahepatic recurrence. Patients considered to be suitable transplant candidates were referred to a transplant centre for further evaluation.
Statistics
Disease and treatment characteristics were summarized using the frequency and percentage for categorical covariates, and the median and range for continuous covariates, and compared using Fisher’s exact test and the Wilcoxon rank-sum test. Cumulative incidences of recurrence within the MC, recurrence beyond the MC and death without recurrence were estimated using competing risks methods and compared using Gray’s test, and Fine and Gray regression. A composite variable representing the presence of either microsatellites based on pathology or multiple tumours based on preoperative imaging was created because it was thought that definitions of each of these may be subjective but the presence of either was more objective. Variables significantly associated with recurrence beyond the MC on univariate analysis were entered into a multivariate model, and backward elimination was used to reduce the model size. In the final model, the hazard ratios associated with recurrence beyond the MC for all three remaining significant risk factors (initial disease beyond the MC, microsatellites or multiple tumours, and lymphovascular invasion) were very similar (ranging from 2.22 to 2.48), and this allowed the development of a simple clinical risk score (CRS) in which 1 point was assigned to each risk factor without weighting, which resulted in the creation of four distinct risk groups. Overall survival and RFS were estimated using Kaplan–Meier methodology and compared using the log-rank test. All statistical analyses were performed using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA) or R Version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria) using the cmprsk package. All P-values were two-sided. P-values of <0.05 were considered to indicate statistical significance.
Results
Clinicopathological characteristics
The study cohort consisted of 320 consecutive patients who underwent elective partial hepatectomy for HCC with negative surgical margins between January 1992 and January 2012 at MSKCC. After the exclusion of patients who died (n = 13; eight before 2000) or were lost from follow-up (n = 3) within 90 days, 304 patients remained for cohort analysis and 295 patients for recurrence pattern analysis (nine patients were of unknown recurrence status). The median age of the patients was 67 years (range: 14–89 years) and 89 (29%) were female. The majority of patients were of ASA class 2 status (48%) or class 3 or 4 status (51%). Median preoperative AFP was 21 ng/ml (range: 1–374 000 ng/ml). Mean preoperative serum bilirubin was 0.7 mg/dl (range: 0.2–11.3 mg/dl) and mean albumin was 4.2 g/dl (range: 2.3–5.1 g/dl). Overall, preoperatively, 210 (69%) patients harboured tumours beyond the MC. A total of 147 (48%) patients underwent a major hepatic resection; the median estimated blood loss was 400 ml (range: 10–8500 ml).
On histopathology, median tumour size was 6.6 cm (range: 0.9–25.5 cm). Most patients had solitary tumours (88%), and were moderately or poorly differentiated (83%). Approximately a quarter of the patients (24%) had cirrhosis. The median follow-up period in event-free patients was 47 months (range: 6−200 months). Tumour recurrence data were available in 295 patients, of whom 183 (62%) developed recurrence; the 5-year cumulative incidence of recurrence was 62.5% for the entire cohort. The cohort characteristics are summarized in Table 1.
Table 1.
Demographic and disease data for patients with preoperative disease within and beyond the Milan criteria (MC)
| Factors | Whole cohort (n = 304) | Within MC groupa (n = 94) | Beyond MC groupa (n = 210) | P-valuea |
|---|---|---|---|---|
| Demographic data | ||||
| Female gender, n (%) | 89 (29%) | 25 (27%) | 64 (30%) | 0.6 |
| Age, years, median (range) | 67 (14–89) | 67 (32–85) | 67 (14–89) | 0.6 |
| MELD score, median (range) | 8 (6–19) | 8 (6–13) | 8 (6–19) | 0.334 |
| Child–Pugh score, median (range) | 5 (5–8) | 5 (5–6) | 5 (5–8) | 0.007 |
| Child–Pugh Grade A, n (%) | 236 (97%) | 70 (100%) | 166 (96%) | 0.1973 |
| Child–Pugh Grade B, n (%) | 7 (3%) | 0 | 7 (4%) | – |
| Child–Pugh Grade C, n (%) | 0 | 0 | 0 | – |
| Operative data | ||||
| Prior liver resection, n (%) | 12 (4%) | 9 (10%) | 3 (1%) | 0.002 |
| Major resection, n (%) | 147 (48%) | 15 (16%) | 132 (63%) | <0.001 |
| Blood loss, ml, median (range) | 400 (10–8500) | 300 (10–3000) | 500 (25–8500) | <0.001 |
| Pathological data | ||||
| Tumour size, cm, median (range) | 6.6 (0.9–25.5) | 3.2 (0.9–13) | 9 (1–25.5) | <0.001 |
| Single tumour, n (%) | 267 (88%) | 86 (91%) | 181 (86%) | 0.3 |
| Differentiation, n (%) | 0.03 | |||
| Well | 51 (17%) | 17 (18%) | 34 (17%) | |
| Moderate | 168 (57%) | 60 (65%) | 108 (53%) | |
| Poor | 77 (26%) | 15 (16%) | 62 (30%) | |
| Lymphovascular invasion, n (%) | 141 (46%) | 32 (34%) | 109 (52%) | 0.004 |
| Microsatellites present, n (%) | 63 (21%) | 13 (14%) | 50 (24%) | 0.05 |
| Cirrhosis present, n (%) | 74 (24%) | 35 (37%) | 39 (19%) | <0.001 |
| Within Milan criteria by pathology | – | 88 (94%) | 19 (9%) | <0.001 |
| Survival, months, median (range) | – | 102.1 (67.3–127.0) | 44.9 (36.1–63.5) | <0.001 |
| RFS, months, median (range) | – | 45.6 (28.8–66.2) | 15.0 (10.2–22.1) | <0.001 |
| Aetiology of primary liver disease, n (%) | <0.001 | |||
| Alcohol | 32 (11%) | 14 (15%) | 18 (9%) | |
| Haemochromatosis | 6 (2%) | 2 (2%) | 4 (2%) | |
| Hepatitis B | 79 (26%) | 29 (31%) | 50 (24%) | |
| Hepatitis B and C | 1 (0.3%) | 1 (1%) | 0 | |
| Hepatitis C | 52 (17%) | 26 (28%) | 26 (12%) | |
| Non-alcoholic steatohepatitis (NASH) | 1 (0.3%) | 0 | 1 (0.5%) | |
| None | 133 (44%) | 22 (23%) | 111 (53%) | |
| Recurrence pattern, n (%) | NA | |||
| Recurrence within MC | – | 27 (29%) | 45 (22%) | |
| Recurrence beyond MC (extrahepatic recurrence) | – | 17 (19%) | 94 (46%) | |
| (11/17) | (63/94) | |||
| No evidence of disease | – | 48 (52) | 64 (32%) | |
| Unknown recurrence status | – | 2 (2%) | 7 (3%) |
The univariate analysis was performed for the within-MC and beyond-MC groups only, not for the whole cohort (whole cohort, n = 304: descriptive data only).
MELD, Model for End-stage Liver Disease; NA, not applicable; RFS, recurrence-free survival.
Preoperative MC status and recurrence patterns
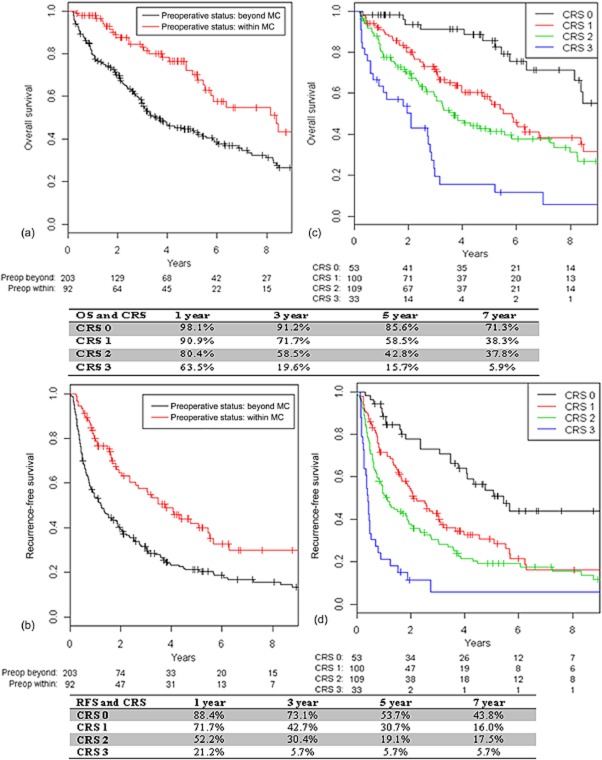
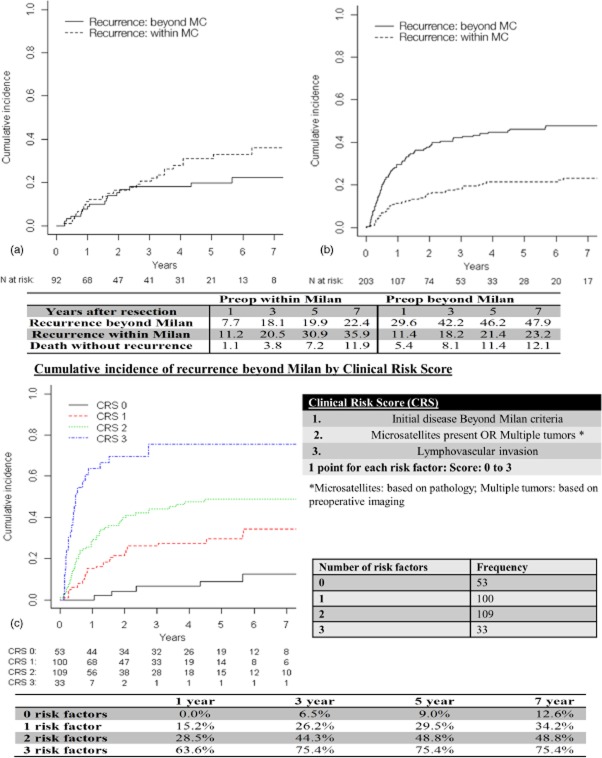
Table 1 represents a comparison of clinical, surgical and pathological data for patients classified with preoperative disease within the MC (n = 94, 31%) or beyond the MC (n = 210, 69%). There was no difference in demographic characteristics between the two groups. Patients of beyond-MC status had larger tumours (9.0 cm versus 3.2 cm; P < 0.001), necessitating major resections more frequently (63% versus 16%; P < 0.001) and, therefore, experienced greater estimated blood loss (500 ml versus 300 ml; P < 0.001). These tumours more often had adverse histopathological characteristics, namely more frequent lymphovascular invasion (52% versus 34%; P = 0.004), microsatellites (24% versus 14%; P = 0.05) and poor differentiation (30% versus 16%; P = 0.03). Figure 1 represents a flow chart of the cohort and the recurrence patterns based on the MC status of patients. Patients with HCC initially within the MC (31% of the cohort) had longer median OS compared with patients of initial beyond-MC status (69% of the cohort) (102 months versus 45 months; P < 0.001) and longer median RFS (46 months versus 15 months; P < 0.001) (Fig. 2a, b). Patients with HCC initially within the MC (31% of the cohort) were more likely to be alive and disease-free at 5 years than those with initial disease beyond the MC (69% of the cohort) (42% versus 21%; P < 0.0001). Patients with HCC initially within the MC were less frequently subject to recurrence beyond the MC (5-year cumulative incidence: 19.9% versus 46.2%) and more frequently subject to recurrence within the MC (5-year cumulative incidence: 30.9% versus 21.4%) than patients with initial disease beyond the MC (Fig. 3a, b).
Figure 1.
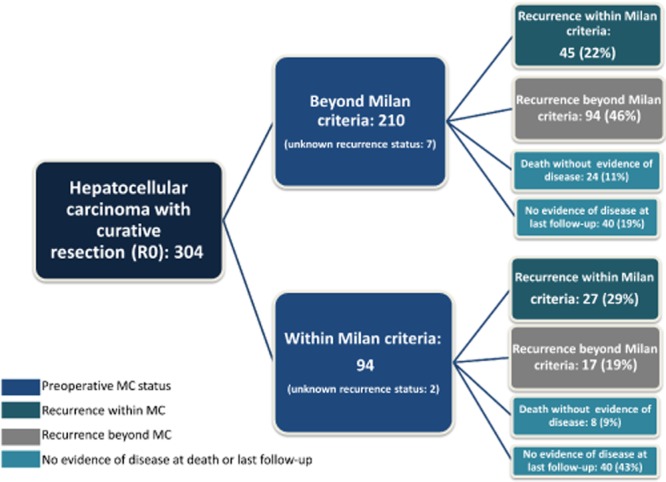
Recurrence patterns according to Milan criteria (MC) status in 304 patients with hepatocellular carcinoma
Figure 2.
Overall survival (OS) and recurrence-free survival (RFS) by initial Milan criteria (MC) status and clinical risk score (CRS) in 295 patients with hepatocellular carcinoma
Figure 3.
Cumulative incidences of recurrence stratified by the Milan criteria (MC) and clinical risk score (CRS) based on preoperative MC status in 295 patients with hepatocellular carcinoma. (a) preoperatively within Milan criteria; (b) preoperatively beyond Milan criteria
In patients who died without evidence of recurrence (n = 32) (Fig. 1), causes of death were liver failure (n = 4), non-HCC malignant disease (n = 7), and medical illness (myocardial infarction, cerebrovascular accident) (n = 3); in 18 patients, the cause of death was multifactorial or unknown.
Treatment of recurrences
Treatment options for HCC recurrences included re-resection, locoregional therapy such as TAE and/or ablation, and systematic therapy with best supportive care. Suitable transplant candidates were referred to a transplant centre for evaluation. This decision-making process was based on a multidisciplinary team discussion.
Of the patients who had recurrences within the MC and for whom good follow-up and treatment data were available (n = 68 of 72 patients with recurrences within the MC), 50% underwent TAE only, 18% underwent re-resection, 16% were treated with combination therapy of TAE and ablation, and 4% underwent ablation only. Overall, five patients (7%) were referred for transplantation evaluation and three (4%) of these were eventually transplanted. Remaining patients (5%) received best supportive care.
Of the patients in whom recurrences were beyond the MC and for whom good follow-up and treatment data were available (n = 65), 48% underwent TAE, 18% received systemic therapy (e.g. sorafenib), 9% underwent re-resection, 5% were treated with a combination of TAE and ablation, and the remaining 20% received best supportive care.
Incorporating clinicopathological characteristics into the MC
Clinical and pathological data are summarized in Table 1. The clinicopathological variables were analysed to predict three different patterns of (non-)recurrence: (i) recurrence within the MC; (ii) recurrence beyond the MC, and (iii) death without recurrence. On univariate analysis, initial disease beyond the MC, gender, major resection, blood loss, tumour size, lymphovascular invasion, microsatellites and the presence of multiple tumours were significantly associated with recurrence beyond the MC. None of the clinicopathological variables including the aetiology of the primary liver disease were significantly associated with recurrence within the MC or death without recurrence (except for age in patients who died without recurrence) (Table 2). In the 94 patients in whom disease was classified as within the MC on preoperative imaging, six (6%) had disease beyond the MC on pathology (pMilan). Of the 210 patients with preoperative disease beyond the MC, 19 (9%) were found to be within pMilan status. The concordance of preoperative MC based on imaging and pMilan was very high, and the statistical significance was preserved in the multivariate analysis regardless of whether preoperative MC or pMilan was used; the effect sizes of preoperative MC and pMilan on recurrence outside the MC were identical. Therefore, preoperative MC status based on imaging was used in the present risk model as it is based on preoperative imaging and is therefore more clinically relevant.
Table 2.
Factors associated with recurrence pattern and death
| Variable | HR comparison | Recurrence beyond MC | Recurrence within MC | Death without recurrence | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR | P-value | ||
| Initial disease beyond MC | Within versus beyond | 0.34 (0.2–0.56) | <0.001 | 1.42 (0.89–2.27) | 0.146 | 0.76 (0.34–1.67) | 0.487 |
| Female | Female versus male | 0.61 (0.39–0.96) | 0.031 | 0.86 (0.52–1.43) | 0.563 | 1.19 (0.58–2.44) | 0.626 |
| Age | Per 1-year increase | 1 (0.98–1.01) | 0.678 | 1 (0.98–1.02) | 0.873 | 1.03 (1–1.06) | 0.042 |
| Prior liver resection | Yes versus no | 0.95 (0.33–2.74) | 0.919 | 1.06 (0.32–3.48) | 0.928 | 0.7 (0.11–4.43) | 0.701 |
| Major resection | Major versus minor | 1.6 (1.1–2.32) | 0.014 | 0.7 (0.44–1.11) | 0.129 | 1.1 (0.55–2.18) | 0.794 |
| Blood loss | Per 100-unit increase | 1.04 (1.03–1.06) | <0.001 | 0.97 (0.93–1.00) | 0.054 | 0.99 (0.96–1.03) | 0.597 |
| α-fetoprotein, ng/ml | <200 versus >200 | 1.48 (0.95–2.28) | 0.080 | 0.97 (0.59–1.6) | 0.899 | 0.67 (0.28–1.56) | 0.347 |
| Tumour size | Per 1-cm increase | 1.11 (1.07–1.16) | <0.001 | 0.96 (0.91–1.01) | 0.096 | 0.99 (0.92–1.06) | 0.687 |
| Solitary tumour | Multiple versus single | 1.87 (1.13–3.1) | 0.016 | 1.14 (0.55–2.36) | 0.727 | 0.75 (0.23–2.4) | 0.630 |
| Differentiation | Well versus poor or moderate | 0.58 (0.33–1.03) | 0.061 | 1.12 (0.61–2.06) | 0.715 | 1.63 (0.75–3.57) | 0.218 |
| Lymphovascular invasion | Yes versus no | 2.38 (1.64–3.45) | <0.001 | 0.75 (0.47–1.2) | 0.230 | 0.72 (0.35–1.47) | 0.367 |
| Microsatellites present | Yes versus no | 2.51 (1.7–3.71) | <0.001 | 0.92 (0.5–1.67) | 0.780 | 0.68 (0.26–1.79) | 0.435 |
| Cirrhosis present | Yes versus no | 1.16 (0.76–1.78) | 0.490 | 1.08 (0.65−1.79) | 0.759 | 1.9 (0.94–3.85) | 0.072 |
| Microsatellites OR multiple tumours | Yes versus no | 2.51 (1.72–3.66) | <0.001 | 0.95 (0.55–1.63) | 0.843 | 0.62 (0.26–1.5) | 0.290 |
| Primary liver diseasea | 0.173 | 0.106 | 0.491 | ||||
| Alcohol-related | Yes versus no | 0.48 (0.23–1) | 2.18 (1.06–4.49) | 2.03 (0.79–5.22) | |||
| Hepatitis B (Hep B) | Hep B versus none | 0.7 (0.43–1.12) | 1.77 (0.98–3.19) | 1.21 (0.51–2.86) | |||
| Hepatitis C (Hep C) | Hep C versus none | 0.65 (0.37−1.14) | 2.05 (1.05–3.98) | 1.15 (0.41–3.26) | |||
| Haemochromatosis (HC) | HC versus none | 1.02 (0.35−2.95) | 0.95 (0.12–7.47) | 0 | |||
| bInitial disease beyond MC | Beyond versus within | 2.46 (1.48–4.11) | <0.001 | 1.35 (0.82–2.22) | 0.241 | 0.66 (0.29–1.48) | 0.312 |
| bMicrosatellites OR multiple tumours | Yes versus no | 2.28 (1.52–3.4) | <0.001 | 1.01 (0.58–1.76) | 0.965 | 0.6 (0.24–1.47) | 0.264 |
| bLymphovascular invasion | Yes versus no | 2.22 (1.52–3.23) | <0.001 | 0.8 (0.48–1.32) | 0.380 | 0.69 (0.33–1.43) | 0.319 |
Rows in bold are to distinguish from the above rows as the important multivariate analysis part that leads to the clinical risk score (CRS).
Patients with non-alcoholic steatohepatitis and combined Hep B/C are excluded.
Multivariate analysis of risk factors associated with recurrence pattern.
95% CI, 95% confidence interval; HR, hazard ratio; MC, Milan criteria.
A multivariate analysis was performed based on the significant risk factors. Initial disease beyond the MC, and the presence of microsatellites or multiple tumours and lymphovascular invasion remained independent risk factors for the prediction of recurrence beyond the MC (Table 2). Other important known risk factors, such as AFP and tumour differentiation, which were of borderline significance (P < 0.1), were each added to the final model but were not independent predictors and were therefore eliminated.
Risk model for the prediction of recurrence beyond the MC
Based on this multivariate analysis, a CRS was devised to predict the recurrence of HCC beyond the MC. The cumulative incidences of recurrence beyond the MC in these risk groups are shown in Fig. 3c. Patients with a score of 0 had 1-, 3- and 5-year incidences of recurrence beyond the MC of 0%, 6.5% and 9.0%, respectively, whereas patients with a score of 3 had significantly higher 1-, 3- and 5-year incidences of recurrence beyond the MC of 63.6%, 75.4% and 75.4%, respectively (P < 0.0001). This analysis was repeated for the cohort of patients of within-MC status, in whom the CRS was found to be significantly associated with a higher incidence of recurrence beyond the MC (P = 0.002) (Fig. S1, online)
Based on this CRS, patients with a score of 0 had 1-, 3- and 5-year OS rates of 98.1%, 91.2% and 85.6%, respectively, which were significantly better than OS in patients with a CRS of 3 (all the risk factors), who achieved 1-, 3- and 5-year OS of 63.5%, 19.6% and 15.7%, respectively (Fig. 2c). Patients with 0 points had 1-, 3- and 5-year RFS of 88.4%, 73.1% and 53.7%, respectively, whereas patients with a CRS of 3 (all the risk factors) had 1-, 3- and 5-year RFS of 21.2%, 5.7% and 5.7%, respectively (Fig. 2d).
Discussion
Liver resection and OLT form the foundation of HCC treatment. Although LR is the preferred treatment modality for resectable tumours in patients with preserved liver function, high recurrence rates remain problematic.25 Liver transplantation, by contrast, is considered the gold standard treatment in patients with cirrhosis with tumours within the MC, but is limited by organ availability, tumour progression while on the transplant waiting list and the problems inherent in the use of lifelong immunosuppression.3 The roles of LR and OLT in HCC are continuously evolving, and there is ongoing debate with regard to the optimal treatment strategy in patients with mild cirrhosis and early HCC.4,26 The controversy between LR and OLT concerns the group of patients with HCC within the MC and limited underlying parenchymal disease (e.g. Child–Pugh class A/low B liver cirrhosis).25,27 Many studies have tried to address this question; unfortunately, because of the retrospective nature and the inherent biases of these studies, cohorts of LR and OLT patients are often not equivalent in terms of clinicopathological characteristics and no strong conclusions have been drawn. A meta-analysis looking at 62 studies comparing LR and OLT in the treatment of HCC reported similar 1-year OS, but patients submitted to OLT achieved significantly better 3- and 5-year OS (when organs were available); the authors also found that disease-free survival and recurrence were similar in early HCC patients with Child–Pugh class A cirrhosis.28 Another meta-analysis by Dhir et al., which looked at 24 studies with a total of 1763 patients with HCC within the MC who were treated with LR versus OLT, echoed the survival benefit of OLT over LR, but this survival advantage disappeared when intention-to-treat analysis was used.29
The recurrence of HCC after LR remains a major limitation of resection in comparison with OLT.25 The strategy of using upfront LR in all resectable HCC in patients with normal or limited liver disease, followed by ST as a back-up option when the tumour recurs, is feasible. It combines the benefits of a good quality of life after LR with survival outcomes similar to those in patients who undergo initial OLT, the lack of a waiting time/dropout factor, is potentially curative, implies less need for longterm immunosuppression, and, perhaps most importantly from a medicosocial standpoint, allows for the more optimal use of donor livers, thereby reserving this scarce resource for patients with no alternative options.11,15 We have previously reported our experience in relation to patterns of recurrence of HCC and have identified tumour size and vascular invasion as predictors of recurrence.30 In a subsequent study, we reported size, vascular invasion and satellitosis to be important in nomograms for recurrence and survival.31 Many other studies and systematic reviews have similarly emphasized the importance of clinicopathological features, such as tumour size and number, time to recurrence, and the presence of micro- or macrovascular invasion as independent predictive factors for recurrence and survival.32–37 Some studies have specifically identified microvascular invasion, poor differentiation, tumour size of >6 cm and portal vein invasion as predictors of the recurrence of HCC beyond the MC. In the present study, initial disease beyond the MC, the presence of microsatellites or multiple tumours and lymphovascular invasion were also found to be independent predictors of the recurrence of HCC beyond the MC. However, the present analyses have failed to identify any risk factors associated with recurrence within the MC (Table 2).
Some centres have advocated prophylactic, pre-emptive listing for ST (i.e. before recurrence is detected), especially in patients with a high risk profile (vascular invasion and/or additional nodules) as recommended by the Barcelona Clinic Liver Cancer (BCLC) group, whereas others have adopted a wait-and-see approach, reserving ST only when recurrences are detected.14,38–41 There is no consensus on the timing of ST after LR. Performing a prophylactic ST before any evidence of tumour recurrence eliminates the chance of recurrence beyond the MC, but subjects the patient to another major procedure and its attendant risks while limiting the benefits of the initial LR in patients without recurrence. It also imposes a significant burden on the limited pool of donated organs. In the present study, the majority of patients would not have benefited from a prophylactic ST: by 5 years post-surgery, only 46% of patients with initial tumours beyond the MC and 20% of patients with initial tumours within the MC had recurrence beyond the MC (Fig. 3). Conversely, the proponents of pre-emptive ST would argue that almost half of patients with HCC recurrence following LR will not make it to ST as a result of various transplant eligibility considerations, including patient factors such as advanced age, patient refusal and tumour factors such as recurrent disease beyond the MC.11 In the USA, the concept of prophylactic transplant post-LR is not applicable because no tumour exception points can be allocated to patients without recurrence after a potentially curative HCC resection.
The present study analysed the factors that can help predict the pattern of HCC recurrence, specifically recurrence beyond the MC, as this group of patients is disadvantaged by the loss of opportunity for OLT, especially those in whom initial tumours were within the MC (and who were thus eligible for initial OLT). By 5 years, 19.9% of patients with initial disease within the MC experienced recurrence beyond the MC, which is similar to the rates of 12–27% reported by other groups.11,21,22 Most of the other studies reported on populations of patients with cirrhosis and tumours within the MC.11 However, the present study examined the potential for ST in a population of patients with advanced tumours arising largely in non-cirrhotic liver, and focused on recurrence patterns in relation to preoperative MC status and clinicopathological risk factors. The present findings demonstrate that a significant percentage of patients who harboured tumours within or beyond the MC remained disease-free or experienced recurrences within the MC, and therefore could be considered for ST. Furthermore, the incorporation of the risk factors into the MC enhanced the identification of patients with unfavourable tumour biology who might potentially benefit from early referral to a transplant centre for consideration for ST after LR rather than the blind adoption of a wait-and-see policy. Other studies have also suggested that the strategy of utilizing pathological factors found at resection to offer ST is feasible.11,13 Fuks et al. found that adverse histopathological characteristics (vascular invasion, satellites, tumour size of >3 cm, poor tumour differentiation and liver cirrhosis) were associated with non-transplantability at recurrence and that these characteristics might be used to better select appropriate patients for ST.11 It should be noted that in the cohort reported by Fuks et al.,11 all patients harboured tumours within the MC and 89% had cirrhosis, whereas the present study comprised patients with advanced disease and healthier normal livers (69% beyond the MC and 24% with cirrhosis). Nonetheless, a significant proportion of recurrences in the present series were within the MC, even in patients with advanced disease initially beyond the MC; therefore, it is not surprising that others have found that in the context of small, transplantable HCC lesions, a significant proportion of patients experienced no recurrence or recurrence within criteria for transplantation.15,18 In patients with a high likelihood of recurrence beyond the MC, the issue of whether they would be better served with upfront OLT or would not benefit greatly from OLT because of underlying aggressive tumour biology is controversial. Furthermore, it is unclear whether patients with a high likelihood of beyond-MC recurrence would be better served by upfront OLT or whether the underlying aggressive tumour biology would limit the benefit of this approach. There is limited evidence to date supporting either theory.6,28 This theoretical debate is further confounded by data showing that the yield of OLT over LR is directly related to waiting time.15,42
The CRS established in the present study is useful for estimating patterns of recurrence of HCC; it incorporates the established risk factors and provides a simple clinical tool that is applicable to patients undergoing resection. It considers a patient’s clinicopathological variables and can provide more accurate predictions of post-resection recurrence than those based solely on clinical judgement or the classification of patients into broad high- and low-risk groups. The CRS will need to be further validated in larger studies.
The limitations of the present study relate to its retrospective nature and the fact that the study population was derived from a single institution. As a comprehensive cancer centre, this institution does not have a solid organ transplantation programme and thus lacks a comparable cohort of patients with HCC who can be listed for OLT at the time of initial presentation. For the same reason, detailed information on ST rates in the present cohort is not available. Nonetheless, the decision-making process at this institution is based on consensus achieved during weekly multidisciplinary conferences and suitable patients for whom OLT is considered a better option are referred to a liver transplantation centre. Additionally, the database at this institution is prospectively maintained, which minimizes the occurrence of errors in its data. There is an element of lead time bias as it is possible that the earlier detection of recurrence through frequent and closer surveillance scans in patients with disease of a worse prognosis might bias the effects of some prognostic factors, causing them to appear more unfavourable. However, follow-up was fairly uniform in this cohort and hence this effect was negligible and is not expected to alter the present results and conclusions.
In summary, in a modern series of LR with curative intent for advanced HCC in a largely non-cirrhotic population, a significant proportion of patients were found to experience recurrence within the MC or to remain disease-free, and thus were eligible for ST. The incorporation into the MC of lymphovascular invasion and the presence of microsatellitosis or multiple tumours further enhanced their prognostic role and should be taken into account when patients are considered for ST at the time of surgical resection and to guide closer surveillance post-resection.
Conflicts of interest
None declared.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's website.
Cumulative incidence of recurrence beyond the Milan criteria by number of risk factors among patients with initial disease within the Milan criteria.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kluger MD, Cherqui D. Surgical management of hepatocellular carcinoma. In: Kee ST, Murthy R, Madoff DC, editors. Clinical Interventional Oncology: Management and Practice. 1st edn. Philadelphia, PA: Elsevier; 2014. pp. 66–76. [Google Scholar]
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(Suppl. 2):44–57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- Pelletier SJ, Fu S, Thyagarajan V, Romero-Marrero C, Batheja MJ, Punch JD, et al. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009;15:859–868. doi: 10.1002/lt.21778. [DOI] [PubMed] [Google Scholar]
- Dutkowski P, De Rougemont O, Mullhaupt B, Clavien PA. Current and future trends in liver transplantation in Europe. Gastroenterology. 2010;138:802–809. doi: 10.1053/j.gastro.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology. 2012;55:132–140. doi: 10.1002/hep.24680. [DOI] [PubMed] [Google Scholar]
- Cillo U, Vitale A, Grigoletto F, Gringeri E, D’Amico F, Valmasoni M, et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am J Transplant. 2007;7:972–981. doi: 10.1111/j.1600-6143.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- Scatton O, Zalinski S, Terris B, Lefevre JH, Casali A, Massault PP, et al. Hepatocellular carcinoma developed on compensated cirrhosis: resection as a selection tool for liver transplantation. Liver Transpl. 2008;14:779–788. doi: 10.1002/lt.21431. [DOI] [PubMed] [Google Scholar]
- Sala M, Fuster J, Llovet JM, Navasa M, Sole M, Varela M, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl. 2004;10:1294–1300. doi: 10.1002/lt.20202. [DOI] [PubMed] [Google Scholar]
- Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899–906. doi: 10.1053/he.2000.5763. [DOI] [PubMed] [Google Scholar]
- Belghiti J, Carr BI, Greig PD, Lencioni R, Poon RT. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15:993–1000. doi: 10.1245/s10434-007-9787-8. [DOI] [PubMed] [Google Scholar]
- Del Gaudio M, Ercolani G, Ravaioli M, Cescon M, Lauro A, Vivarelli M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant. 2008;8:1177–1185. doi: 10.1111/j.1600-6143.2008.02229.x. [DOI] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Longterm survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: results of downstaging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Suzuki T, et al. Recurrence patterns after hepatectomy of hepatocellular carcinoma: implication of Milan criteria utilization. Ann Surg Oncol. 2009;16:1560–1571. doi: 10.1245/s10434-009-0407-7. [DOI] [PubMed] [Google Scholar]
- Hung HH, Lei HJ, Chau GY, Su CW, Hsia CY, Kao WY, et al. Milan criteria, multi-nodularity, and microvascular invasion predict the recurrence patterns of hepatocellular carcinoma after resection. J Gastrointest Surg. 2013;17:702–711. doi: 10.1007/s11605-012-2087-z. [DOI] [PubMed] [Google Scholar]
- Ito K, Ito H, Are C, Allen PJ, Fong Y, DeMatteo RP, et al. Laparoscopic versus open liver resection: a matched-pair case control study. J Gastrointest Surg. 2009;13:2276–2283. doi: 10.1007/s11605-009-0993-5. [DOI] [PubMed] [Google Scholar]
- Strasberg SM, Phillips C. Use and dissemination of the Brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257:377–382. doi: 10.1097/SLA.0b013e31825a01f6. [DOI] [PubMed] [Google Scholar]
- Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, et al. Liver resection for transplantable hepatocellular carcinoma: longterm survival and role of secondary liver transplantation. Ann Surg. 2009;250:738–746. doi: 10.1097/SLA.0b013e3181bd582b. [DOI] [PubMed] [Google Scholar]
- Turcotte S, Dematteo RP. Resection versus transplantation for early hepatocellular carcinoma: more art than science. Ann Surg. 2012;256:892–893. doi: 10.1097/SLA.0b013e318275a183. [DOI] [PubMed] [Google Scholar]
- Can MF, Hughes CB. Primary liver transplantation vs liver resection followed by transplantation for transplantable hepatocellular carcinoma: liver functional quality and tumour characteristics matter. World J Gastrointest Surg. 2013;5:5–8. doi: 10.4240/wjgs.v5.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Liang W, Milgrom DP, Schroder PM, Kong NS, Yang C, et al. Liver transplantation versus liver resection in the treatment of hepatocellular carcinoma: a meta-analysis of observational studies. Transplantation. 2014;97:227–234. doi: 10.1097/TP.0b013e3182a89383. [DOI] [PubMed] [Google Scholar]
- Dhir M, Lyden ER, Smith LM, Are C. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: a meta-analysis. HPB. 2012;14:635–645. doi: 10.1111/j.1477-2574.2012.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–758. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Cho CS, Gonen M, Shia J, Kattan MW, Klimstra DS, Jarnagin WR, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg. 2008;206:281–291. doi: 10.1016/j.jamcollsurg.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma – a systematic review. Surg Oncol. 2013;22:e23–e30. doi: 10.1016/j.suronc.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622–1629. doi: 10.1002/bjs.8915. [DOI] [PubMed] [Google Scholar]
- Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumour recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Kakisaka T, et al. Analysis of the risk factors for early death due to disease recurrence or progression within 1 year after hepatectomy in patients with hepatocellular carcinoma. World J Surg Oncol. 2012;10:107. doi: 10.1186/1477-7819-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazaki K, Matsushita A, Nakagawa K, Misawa R, Amano J. Risk factors of longterm survival and recurrence after curative resection of hepatocellular carcinoma. Hepatogastroenterology. 2005;52:552–557. [PubMed] [Google Scholar]
- Yao FY, Hirose R, LaBerge JM, Davern TJ, Bass NM, Kerlan RK, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- Majno P, Giostra E, Mentha G. Management of hepatocellular carcinoma on the waiting list before liver transplantation: time for controlled trials? Liver Transpl. 2007;13(Suppl):27–35. doi: 10.1002/lt.21328. [DOI] [PubMed] [Google Scholar]
- Majno P, Mentha G, Mazzaferro V. Resection, transplantation, either, or both? Other pieces of the puzzle. Liver Transpl. 2005;11:1177–1180. doi: 10.1002/lt.20495. [DOI] [PubMed] [Google Scholar]
- Hwang S, Moon DB, Lee SG. Liver transplantation and conventional surgery for advanced hepatocellular carcinoma. Transpl Int. 2010;23:723–727. doi: 10.1111/j.1432-2277.2010.01103.x. [DOI] [PubMed] [Google Scholar]
- Sarasin FP, Giostra E, Mentha G, Hadengue A. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology. 1998;28:436–442. doi: 10.1002/hep.510280222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cumulative incidence of recurrence beyond the Milan criteria by number of risk factors among patients with initial disease within the Milan criteria.