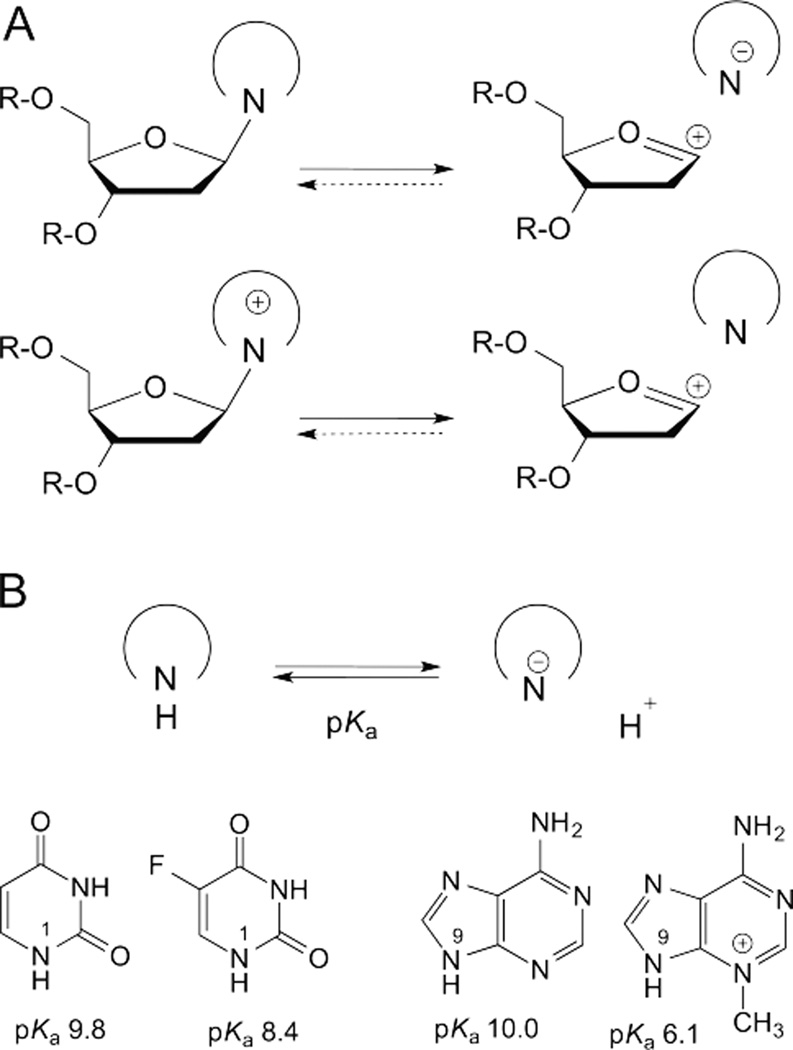
Fig. 4.
Leaving group quality of the nucleobase depends on the acidity (pKa) of its glycosidic nitrogen. (A) In the stepwise reactions for glycosidic bond hydrolysis, a neutral base departs as an anion and a cationic base departs as a neutral species. (B) Acidity of the glycosidic nitrogen for a given base depends on the stability of the N-deprotonated species (conjugate base). The glycosidic nitrogen is N1 for pyrimidines, N9 for purines. Acidity (pKa) is shown for U, 5FU, A, and 3-methyl-A.