Fig. 7.
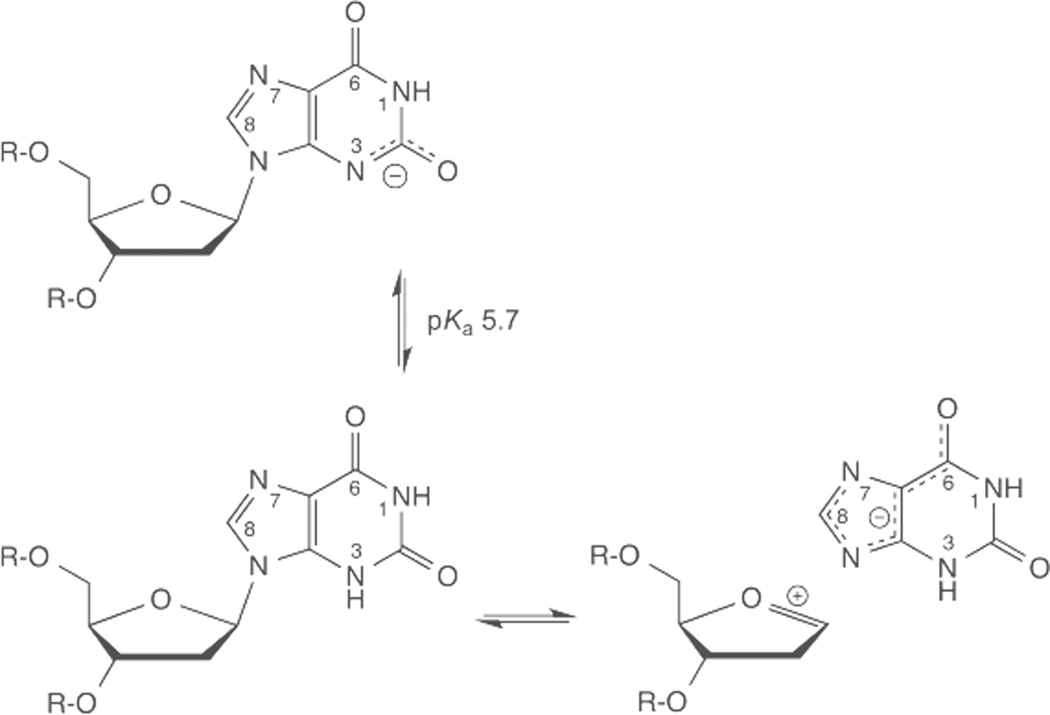
2’-deoxyxanthosine (dX) ionizes at N3 with a pKa of about 5.7 (based on xanthosine) and is predominantly anionic at pH 7.4. Hydrolysis of neutral dX results in departure of the xanthine (X) monoanion, which should be a good leaving group, given that N9 is acidic for X relative to other purines, due perhaps to charge delocalization (N7, O6).