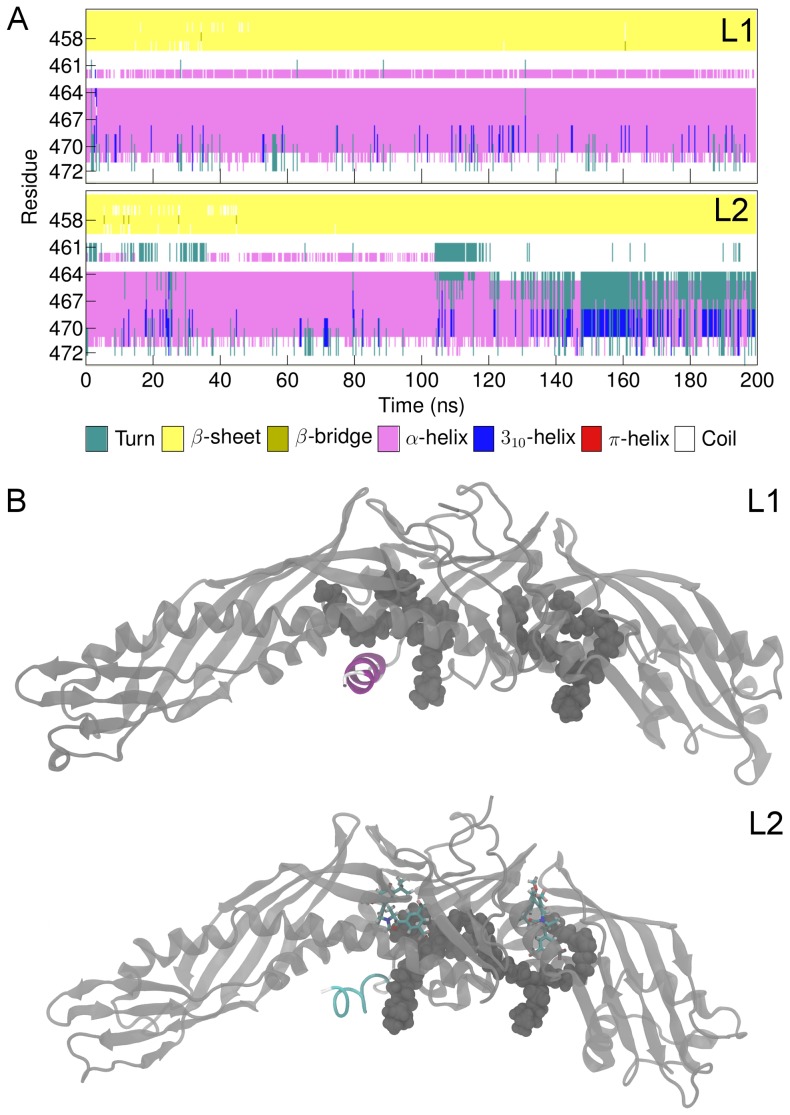
Figure 9. Conformational fluctuations of helix X.
A) Secondary structure of helix X (residues 461–472) during simulations L1 and L2. B) Simulation snapshots from simulations L1 and L2. Helix X is colored to highlight the changes in the secondary structure. In the absence of anacetrapib (L1), helix X maintains the α-helical structure, whereas in the presence of anacetrapib (L2) it alternates between turn (unfolding of the helix) and 310-helix (extension of the helix). Turn-like conformation is presented in the figure.