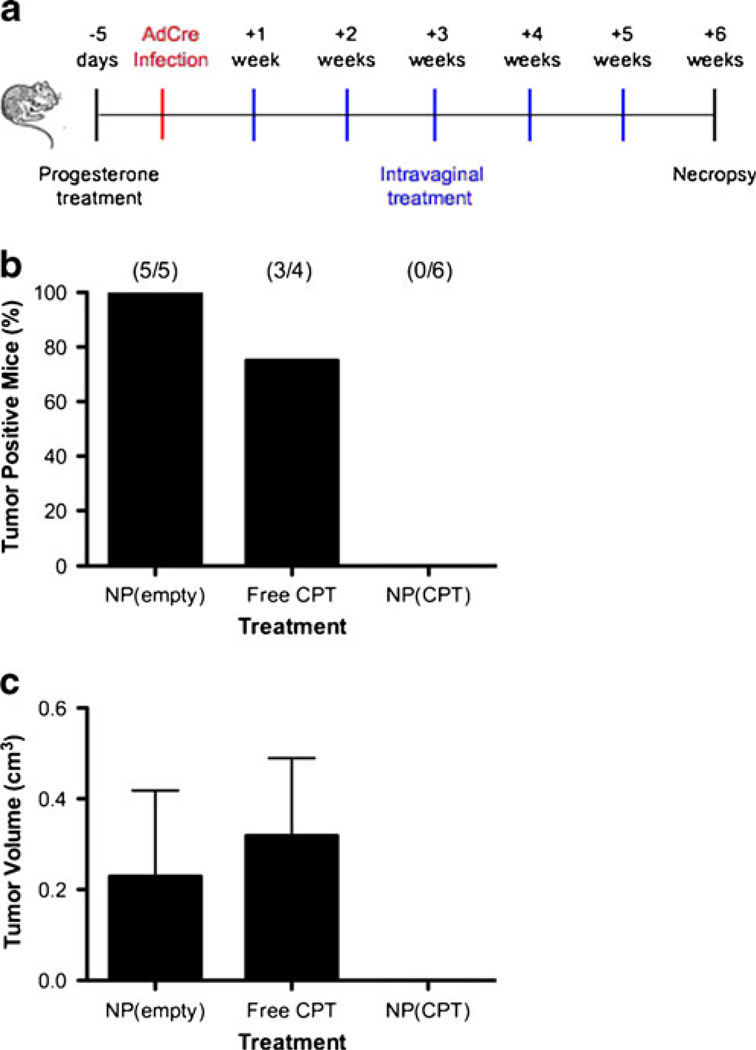
Fig. 4.
Treatment of intravaginal tumors by CPT-loaded nanoparticles. a Experimental design for evaluating nanoparticle efficacy against intra-vaginal tumors: (1) Depo-Provera was administer 5 days prior to infection; (2) animals were infected by intravaginal lavage with AdCre to induce tumors; (3) 1 week post infection animals began receiving weekly treatments with blank nanoparticles, free CPT, or CPT-loaded nanoparticles for a period of 5 weeks; (4) 6 weeks post infection animals were euthanized and evaluated for the presence of intravaginal tumors. b Number of mice per group with observable intravaginal tumors after treatment with blank nanoparticles, free CPT, or CPT-loaded nanoparticles. c Average gross tumor volume per group after treatment with blank nanoparticles, free CPT, or CPT-loaded nanoparticles. Volumes do not include mice that did not develop gross tumors