Abstract
The ovine sexually dimorphic nucleus (oSDN) is 2 times larger in rams than in ewes. Sexual differentiation of the oSDN is produced by testosterone exposure during the critical period occurring between gestational day (GD)60 and GD90 (term, 147 d). We tested the hypothesis that testosterone acts through the androgen receptor to control development of the male-typical oSDN. In experiment 1, pregnant ewes received injections of vehicle, androgen receptor antagonist flutamide, or nonaromatizable androgen dihydrotestosterone (DHT) propionate during the critical period. Fetuses were delivered at GD135. Both antagonist and agonist treatments significantly reduced mean oSDN volume in males but had no effects in females. Experiment 2, we analyzed the effect of treatments on the fetal hypothalamic-pituitary-gonadal axis to determine whether compensatory changes in hormone secretion occurred that could explain the effect of DHT. Pregnant ewes were injected with vehicle, flutamide, or DHT propionate from GD60 to GD84, and fetuses were delivered on GD85. Flutamide significantly increased LH and testosterone in males, whereas DHT significantly decreased both hormones. In females, LH was unaffected by flutamide but significantly reduced by DHT exposure. DHT significantly decreased pituitary gonadotropin and hypothalamic kisspeptin mRNA expression in males and females. These results suggest that androgen receptor mediates the effect of testosterone on oSDN masculinization, because this process was blocked by the androgen receptor antagonist flutamide in eugonadal males. In contrast, the reduction of oSDN volume observed after DHT exposure appears to be mediated by a negative feedback mechanism exerted on the hypothalamus to reduce LH and testosterone secretion. The reduced androgen exposure most likely accounted for the decreased oSDN volume. We conclude that, during the critical period, the male reproductive axis in long gestation species, such as sheep, is sufficiently developed to react to perturbations in serum androgens and mitigate disruptions in brain masculinization.
There is a morphological sex difference within the medial preoptic area (MPOA) of the sheep brain that is referred to as the ovine sexually dimorphic nucleus (oSDN). The oSDN was first described in adult sheep (1) and subsequently shown to be present in fetal lambs (2). It constitutes a dense cluster of neuron in the central component of the medial preoptic nucleus that is identified by strong expression of aromatase mRNA and on average is 2 times larger in males than in females. Approximately 8% of adult rams prefer to mount other rams instead of ewes, and this preference is linked to a smaller oSDN similar in volume to that of ewes. In sheep, the critical period for sexual differentiation occurs from gestational day (GD)30 to GD90 of a 147-day pregnancy and depends largely on the hormonal environment (3). Differentiation of the primordial gonad into a testis begins around GD30, and blood levels of testosterone (T) rise significantly in males during this period (4–6). Initial studies demonstrated that treatment of female fetuses with T for the duration of the critical period stimulates formation of male genitals and increases the volume of the oSDN (2). Subsequently, it was found that these different aspects of sexual differentiation take place during separate critical periods, with male genital development occurring during GD30–GD60 and oSDN growth during GD60–GD90 (7).
Under normal physiological conditions, T is the major sex steroid in the circulation responsible for male sexual differentiation. However, it is well documented that in mammals, including sheep, T is metabolized in the brain to estradiol (E2)via aromatase and/or dihydrotestosterone (DHT) via 5α-reductase (8–11). These metabolites then act through separate estrogen receptor and/or androgen receptor pathways. In other species that also exhibit a SDN in the preoptic area-anterior hypothalamus, such as rats, guinea pigs, and ferrets, the sex difference has been related to the perinatal action of T derived E2 in males (12–14). Studies in sheep suggest that both androgens and estrogens organize aspects of neuroendocrine feedback mechanisms and sexual behaviors (15, 16). There is some evidence that estrogen is involved in masculinization of copulatory behaviors in rams but not required for the formation of the oSDN (17, 18). On this basis, it was hypothesized that androgen activity alone is responsible for the development and differentiation of the male-typical oSDN. Thus, an initial experiment was conducted to study the oSDN in fetal lambs at GD135 after maternal injection of the androgen receptor antagonist flutamide or nonaromatizable androgen agonist DHT propionate from GD60 to GD90.
We coupled this experiment with an analysis of the effects that maternal injections of flutamide and DHT propionate have on the fetal reproductive axis in order to determine which, if any, effects might be secondary to changes in fetal hormone secretions. Previous studies suggest that negative feedback regulation of the hypothalamus-pituitary-gonadal (HPG) axis in fetal sheep is functional during midgestation when the oSDN is developing (19, 20). Thus, a second experiment was performed to measure serum sex steroids and LH concentrations at GD85 after administration of the androgen receptor antagonist and agonist to pregnant ewes from GD60 to GD84. We also quantified effects on the expression of mRNAs coding for subunits of the pituitary gonadotropins. Secretion of LH and FSH depends critically on appropriate pulsatile GnRH secretion by the fetal hypothalamus, which is functional by GD81 (21–24). Recent research has shown that kisspeptin, the protein product of the kisspeptin (KISS1) gene, is vital for steroid regulation of GnRH neurosecretion and timing of puberty (25, 26). Therefore, we determined whether exposure to flutamide and DHT acts centrally to alter KISS1 and/or GnRH mRNA expression in the ovine fetus. We report that disruptions in androgen action during the critical period alter differentiation of the oSDN and lead to changes in fetal hormone secretions.
Materials and Methods
Animals
These studies were conducted according to the principles and procedures outlined by the National Institutes of Health, and all protocols were approved by the Institutional Animal Care and Use Committee at Oregon State University. Polypay ewes (Ovis aries) were obtained from the resident flock at OSU, a facility that is inspected by the United States Department of Agriculture and approved by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Experiment 1. Effect of prenatal exposure to flutamide or DHT propionate on the volume of the oSDN
To investigate the potential effect of an androgen receptor agonist and antagonist on fetal oSDN development, 28 pregnant ewes were treated with either flutamide (AA PharmSyn) or DHT propionate (Steraloids). DHT propionate is a long-acting 17-alkylated derivative of DHT, which is hydrolyzed before acting (27) and measured in serum as DHT. This dose was shown previously to affect aspects of steroid negative feedback control in adults (15, 28). Treatments were conducted from GD60 through GD90. Flutamide (500 mg) was given twice daily by sc injection in 1 mL of dimethyl sulfoxide (DMSO) (n = 10 ewes). DHT propionate (100 mg) was given biweekly by intramuscular injection in 2 mL of corn oil (n = 9 ewes). Ewes assigned to the flutamide group also received corn oil injections, and those assigned to the DHT propionate treatment group also received DMSO vehicle injections. Control (CTL) ewes (n = 9) received both biweekly corn oil and twice daily DMSO injections. Fetuses were delivered surgically on GD135.
Experiment 2. Determine the impact of prenatal exposure to flutamide and DHT propionate on the HPG axis of the fetus
To determine whether maternal injection of flutamide or DHT propionate alters HPG function in the developing fetus, 30 pregnant ewes were treated with vehicle (CTL, n = 8), flutamide (n = 9), or DHT propionate (n = 13) from GD60 to GD84 as described above. Fetuses were delivered surgically on GD85, 24 hours after the final DHT propionate injection.
Blood and fetal tissue collection
Jugular blood samples (5 mL) were drawn from dams. Fetuses were then delivered surgically under anesthesia as described previously (2). In experiment 1, fetal brains were removed from the skull, weighed, and dissected to obtain a diencephalic block of tissue that encompassed the hypothalamus and preoptic area. The tissue was immersion fixed overnight in buffered 4% paraformaldehyde, cryoprotected in 20% sucrose, then frozen and stored at −80°C until sectioned. In experiment 2, fetal brains were weighed and split in half longitudinally along the midline. The right diencephalon was dissected en bloc as described above. The left half of the brain was used to dissect out the MPOA and medial basal hypothalamus (MBH) using anatomical landmarks. The MPOA, MBH, and anterior pituitaries were frozen fresh on dry ice and stored at −80°C for RNA analysis.
In situ hybridization and image analysis
In situ hybridization and thionin staining were used to identify and measure the volume of the developing oSDN (2). Fixed tissues were sectioned coronally (40 μm thick) into parallel series, mounted onto Superfrost microscope slides (Fisher Scientific Co), and stored frozen at −80°C. Adjacent series of brain sections were stained with thionin or processed for in situ hybridization using a sheep-specific [33P] aromatase cRNA as described previously (1). The boundary of the oSDN was defined as the central portion of the medial preoptic nucleus that was most intensely stained with thionin and exhibited the highest density for exposure on film autoradiograms of aromatase mRNA expression. The outline of the oSDN was traced by hand, and the cross-sectional area was measured bilaterally from both thionin-stained sections and film autoradiograms using NIH ImageJ software. Volumes were estimated by multiplying the cross-sectional area and length of the oSDN (ie, number of sections per distance between sections) as described previously (1).
Quantitative reverse transcription real-time PCR
Total RNA was extracted using TRIzol (Invitrogen) and converted to cDNA using the First Strand Superscript III kit (Invitrogen). Real-time PCRs were run in triplicate for each sample using PowerSYBR Green Master Mix (Invitrogen). Primer sets (Supplemental Table 1) for ovine genes were specifically designed to cross exon junctions using Clone Manager software version 8 (Sci-Ed Software). All reactions were run in an ABI Fast 7500 Thermal Cycler (Applied Biosystems, Life Technologies) as described previously (29). The primer efficiencies were more than or equal to 85% for all primer pairs, and all melting curves showed a single peak. Quantification of gene expression was performed by the relative standard curve method (30), normalized against the reference gene glyceraldehyde-3-phosphate dehydrogenase, and reported as the fold difference relative to the mean expression level in the MPOA of pooled GD100 male and female fetuses (GnRH) or in the MBH of pooled GD86 male and female fetuses (KISS1) as previously described (29). Note that no significant variations in glyceraldehyde-3-phosphate dehydrogenase expression were observed between sexes or ages (data not shown).
Serum hormone measurements
Serum steroid concentrations were measured by RIA at the Endocrine Technology and Support Core at the Oregon National Primate Research Center using previously published procedures (31). The sensitivity/tube for E2, T, and DHT was 1, 5, and 5 pg, respectively. The inter- and intraassay variations were less than 10% and 8%, respectively. Serum LH concentrations were measured by RIA, using a modification of Niswender et al (32). The sensitivity/tube averaged 0.12 ng of the NIDDK-oLH-I-4 standard. Intraassay coefficient of variation averaged 4.3% for plasma pools displacing radiolabeled LH to approximately 69% of the total bound, and interassay coefficient of variation was 8.6% using the same plasma pool.
Flutamide and 2-OH-flutamide measurements
Flutamide and its major metabolite, 2-OH-flutamide, were measured in the Pharmacokinetic Core Laboratory at Oregon Health and Science University using a Shimadzu liquid chromatography system interfaced to an Applied Biosystems mass spectrometer equipped with a TurbolonSpray source and Analyst Software. Flutamide and 2-OH-flutamide were obtained from the Schering Corp, and the internal standard (tegafur [5-fluoro-1-(oxolan-2-yl)pyrimidine-2,4-dione]) was obtained from TCI America. Analysis was adapted from Zheng et al (33).
Statistical analyses
Gene expression data and hormone concentrations were analyzed by two-way ANOVA, with post hoc comparisons made using Fisher's least squares difference test. Data were log10 or square root transformed when necessary to equalize variances among groups. All data are expressed as mean ± SEM. Statistical significance was defined as P < .05.
Results
Experiment 1. Effect of maternal injection with flutamide or DHT propionate from GD60 to GD90 on the volume of the oSDN in GD135 lamb fetuses
Maternal injection of flutamide and DHT propionate reduced the volume of the fetal oSDN
Autoradiograms of aromatase mRNA expression matched for position showed labeling in the oSDN of CTL and experimental groups (Figure 1, upper panel). Figure 1, lower panel, shows the effects of flutamide and DHT propionate on oSDN volume in male and female lamb fetuses as defined by the pattern of aromatase mRNA expression. Two-way ANOVA revealed a significant main effect of sex (F(1,35) = 37.5; P < .001), a trend for effect of treatment (F(2,35) = 2.3; P = .1) and a significant sex per treatment interaction (F(2,35) = 4.7; P < .05). Planned post hoc comparisons indicated that oSDN is larger in males than in females. Both flutamide and DHT exposure reduced oSDN volume in males. Neither treatment significantly affected oSDN volume in females, although mean oSDN volume in DHT-exposed females appeared to be slightly enlarged but was not significantly different from DHT- or flutamide-exposed males. There was a comparable effect of sex (F(1,35) = 58.6; P < .001) and treatment (F(2,35) = 6.8; P < .01), and a significant interaction (F(2,35) = 4.0; P < .05), when thionin staining was used to define the borders of the oSDN (Supplemental Figure 1A). The oSDN was longer in males than in females (F(1,35) = 11.2; P < .01), but length was not affected by prenatal hormone exposure (F(2,35) = 0.2; P > .5) (Supplemental Figure 1B). Maternal injection of flutamide and DHT propionate did not affect serum T concentrations, brain weights, or physical growth parameters in lamb fetuses at GD135 (Supplemental Table 2).
Figure 1.
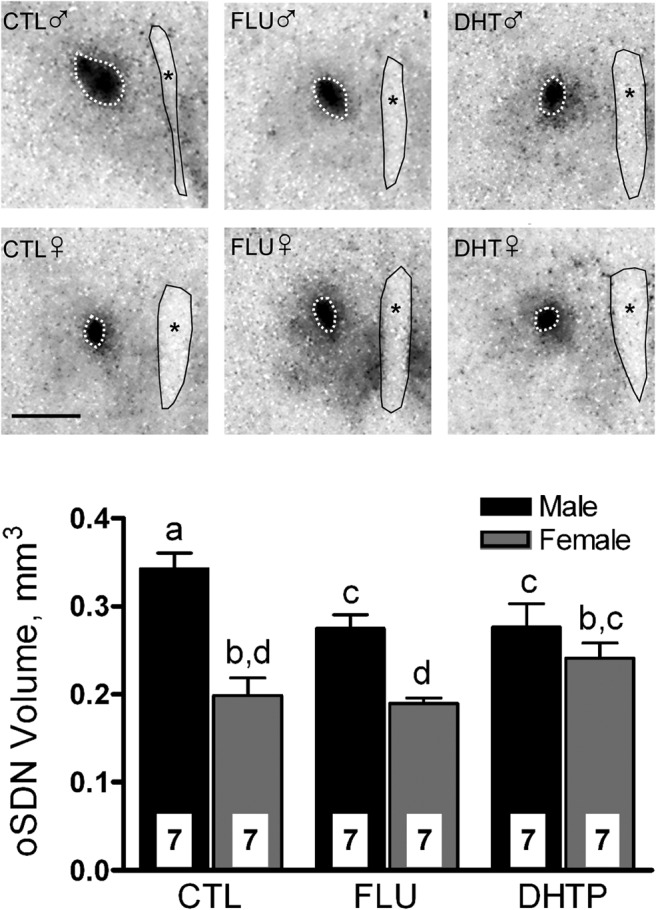
Upper panel, Representative autoradiographic images of aromatase mRNA expression showing coronal sections through the oSDN of GD135 fetuses from CTL, flutamide (FLU), or DHT propionate (DHTP) treatment groups. Image contrast was enhanced to highlight the position of aromatase mRNA expression demarcating the oSDN. *, third ventricle. Scale bar, 1 mm. Lower panel, Effects of maternal injection with vehicle (CTL), FLU, or DHTP from GD60 to GD90 on oSDN development. Data are mean volumes (±SEM) calculated from aromatase mRNA expression and analyzed by two-way ANOVA followed by least squares difference test. Bars with different superscripts differ significantly (P < .05).
Experiment 2. Effects of maternal injection with flutamide and DHT propionate from GD60 to GD84 on the function of the HPG axis in GD85 lamb fetuses
Serum LH and T concentrations in lamb fetuses were enhanced by maternal injection of flutamide and suppressed by maternal injection of DHT propionate
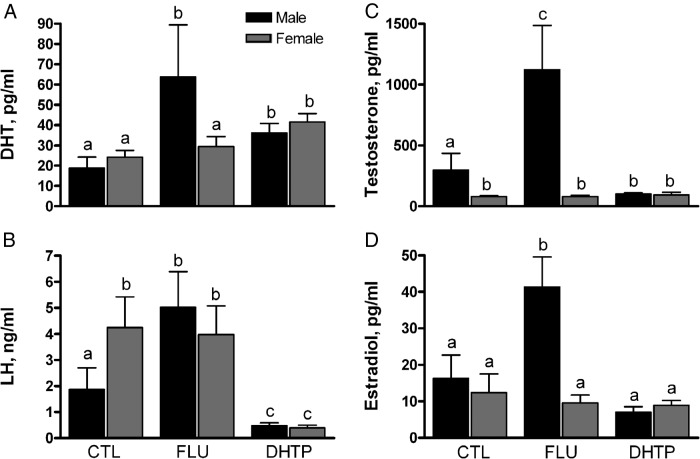
Because flutamide was only partially effective as an antagonist and DHT propionate exposure paradoxically reduced the volume of the oSDN in males, a second experiment was performed to determine whether these treatments altered the secretion of anterior pituitary and gonadal hormones in the midgestation fetus. Maternal injection of flutamide produced elevated concentrations of the antagonist in maternal venous sera (291 ± 23 ng/mL) and fetal umbilical artery sera (73 ± 11 ng/mL). The major active metabolite 2-OH-flutamide was significantly higher in both maternal (1533 ± 49 ng/mL) and fetal (577 ± 35 ng/mL) sera. Neither flutamide nor 2-OH-flutamide was detected in maternal and fetal sera of vehicle-injected CTLs. Maternal injection of DHT propionate significantly (P < .05) elevated sera concentrations of DHT in treated ewes (2.9 ± 0.4 ng/mL) compared with both vehicle- or flutamide-injected ewes (0.1 ± 0.02 and 0.06 ± 0.01 ng/mL, respectively). Similarly, in fetuses, DHT propionate treatment significantly (P < .05) elevated umbilical sera concentrations of DHT in males (36 ± 4.7 pg/mL) and females (42 ± 4.1 pg/mL) compared with vehicle-treated CTL males (19 ± 5.6 pg/mL) and females (24 ± 3.3 pg/mL) (Figure 2A).
Figure 2.
Effects of maternal injections with vehicle (CTL), flutamide (FLU), and DHT propionate (DHTP) from GD60 to GD84 on serum steroid and LH concentrations in GD85 ovine fetuses. Data (mean ± SEM) were analyzed by two-way ANOVA. Bars with different superscripts differ significantly (P < .05).
Changes in fetal hormones levels after flutamide and DHT propionate treatments are shown in Figure 2, A–D. Two-way ANOVA revealed significant main effects of maternal injections of flutamide and DHT propionate on fetal serum LH (F(2,41) = 20.7; P < .0001), DHT (F(2,42) = 5.3; P < .01), T (F(2,43) = 11.8; P < .0001), and E2 (F(2,43) = 4.1; P < .05). In male fetuses, LH, DHT, T, and E2 were significantly increased (P < .05) by flutamide exposure, and LH and T, but not E2, were significantly suppressed (P < .05) by DHT. In female fetuses, flutamide had no effect on gonadal steroid and LH concentrations, and DHT suppressed (P < .05) LH. A significant main effect of sex was observed for T (F(2,43) = 40.2; P < .0001) and E2 (F(2,43) = 5.1; P < .05) but not for DHT and LH. Mean serum concentrations of T were significantly (P < .05) higher in CTL males than in females, and mean LH concentrations were significantly (P < .05) higher in CTL females than in males. Males exhibited significantly (P < .05) higher concentrations of DHT, T, and E2 than females in the flutamide treatment group. Post hoc statistical analyses also revealed significantly (P < .05) greater serum DHT levels in males than in females in response to maternal flutamide injection. Mean testis weights were significantly (P < .05) increased by flutamide and decreased by DHT propionate exposure, whereas ovarian weights were not affected (Figure 3). These data provide evidence that the HPG axis in the male fetus, but not the female, is subject to tonic negative feedback that is disrupted by flutamide and enhanced by DHT.
Figure 3.
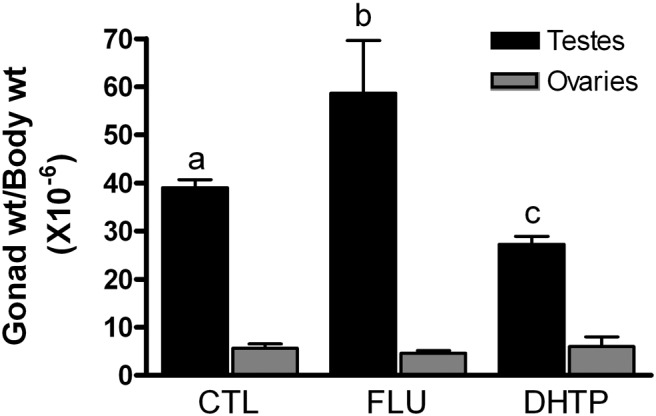
Effects of maternal injections with vehicle (CTL), flutamide (FLU), or DHT propionate (DHTP) from GD60 to GD84 on gonadal weights (wt) in GD85 ovine fetuses. Data (mean ± SEM) were analyzed by one-way ANOVA by sex. Bars with different superscripts differ significantly (P < .05).
Gonadotropin subunit gene expression in fetal lamb pituitaries was suppressed by maternal injection of DHT propionate
Androgens feed back on the anterior pituitary to control gonadotropin secretion and synthesis. Thus, we assessed the effect of antagonist and agonist treatment on gonadotropin gene expression (Figure 4). Two-way ANOVA revealed significant main effects of maternal treatments on the expression of mRNAs for LHβ (F(2,32) = 16.6; P < .0001), FSHβ (F(2,31) = 13.6; P < .0001), and α-subunit (F(2,31) = 3.4; P < .05) in the fetal anterior pituitary. Maternal injection of flutamide had no effects on gonadotropin gene expression. Maternal injection of DHT propionate significantly suppressed (P < .05) LHβ and FSHβ mRNA levels in male fetuses and α-subunit, LHβ, and FSHβ in females. Levels of FSHβ mRNA and α-subunit mRNA were significantly higher in females than in males (F(1,31) = 11.2 and F(1,31) = 9.2; P < .01); no sex difference was found in LHβ mRNA.
Figure 4.
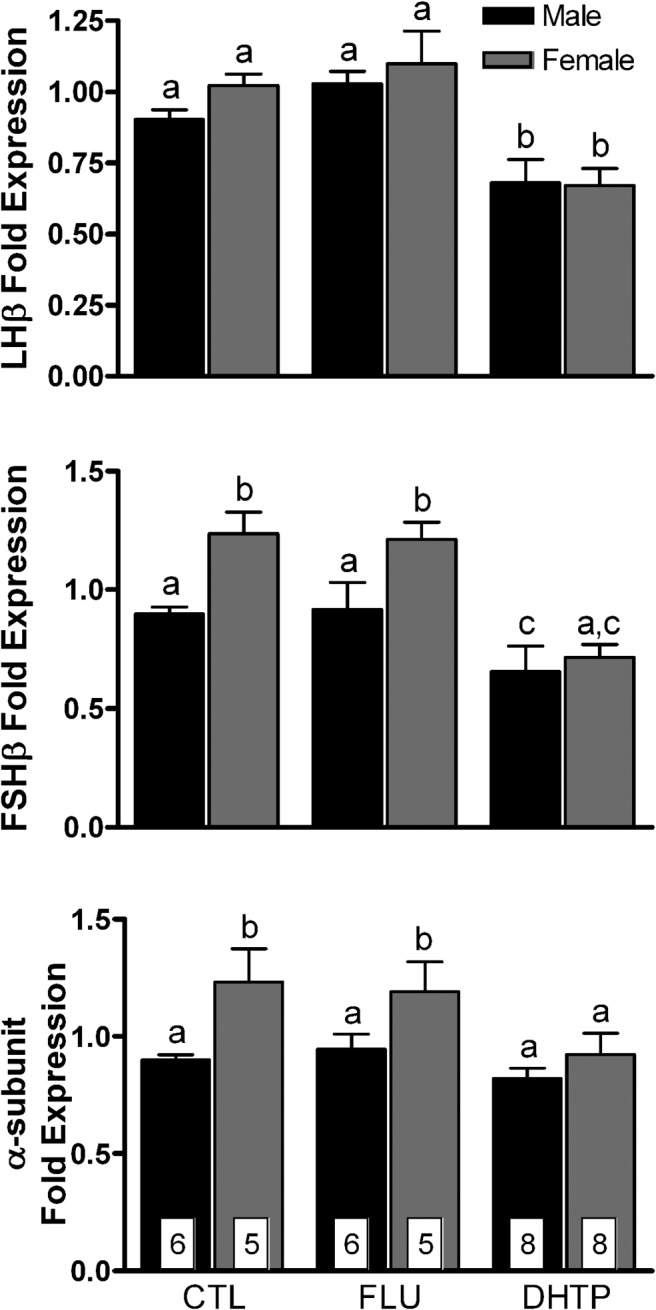
Effects of maternal injections with vehicle (CTL), flutamide (FLU), or DHT propionate (DHTP) from GD60 to GD84 on gonadotropin subunit gene expression in GD85 ovine fetal pituitaries. Data (mean ± SEM) were analyzed by two-way ANOVA. Bars with different superscripts differ significantly (P < .05).
KISS1 mRNA expression in the fetal lamb MBH was suppressed by maternal injection of DHT propionate; no effects were observed on the expression of GnRH mRNA
Figure 5 illustrates the effect of antagonist and agonist treatment on the expression of KISS1 and GnRH mRNA in the fetal lamb hypothalamus. Two-way ANOVA revealed a significant main effect of maternal treatments on KISS1 mRNA expression (F(1,32) = 15.6; P < .0001) and a significant main effect of sex (F(1,32) = 16.5; P < .0001). Planned post hoc comparisons indicated that flutamide had no effect, whereas DHT treatment significantly (P < .05) suppressed KISS1 mRNA expression in both male and female fetuses. Significantly (P < .05) greater KISS1 mRNA expression was found in female than in male fetuses after maternal injections of either flutamide or DHT propionate accounting for the main effect of sex. KISS1 mRNA was not detectable in the fetal MPOA dissection (data not shown).
Figure 5.
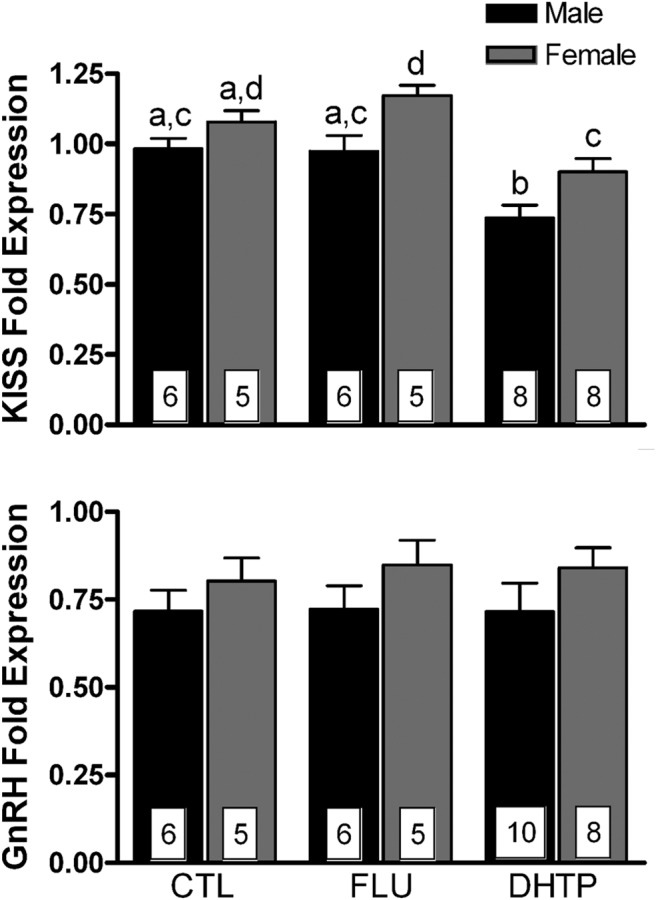
Effects of maternal injections with vehicle (CTL), flutamide (FLU), or DHT propionate (DHTP) from GD60 to GD84 on hypothalamic GnRH and Kiss1 gene expression in GD85 ovine fetuses. Data (mean ± SEM) were analyzed by two-way ANOVA. Bars with different superscripts differ significantly (P < .05).
GnRH mRNA expression was measured in both fetal MBH (Figure 5) and MPOA (Supplemental Figure 2). No significant effects of maternal treatment or sex were found in either tissue. However, there was a trend for GnRH expression to be greater in MBH of females than of males (F(1,34) = 3.3; P = .08).
Maternal injections with flutamide and DHT propionate did not affect the body weight, brain weight, or crown-rump length of exposed fetuses (Supplemental Table 3). The ratio of ano-genital distance to ano-umbilical distance was significantly larger in males than in females (F(1,43) = 10 228.6; P < .0001) but was not affected by treatment (F(2,43) = 0.6; P = .5).
Discussion
This investigation provides convincing evidence that the androgen receptor mediates masculinization of the oSDN in eugonadal male lamb fetuses. Flutamide exposure antagonizes the androgen receptor to directly block the action of endogenous androgen on the oSDN, whereas the effects of DHT exposure inhibit the hypothalamus-pituitary-testis axis, which decreases LH, leading to lower serum T concentrations, both of which reduce the volume of the oSDN in males. Previous studies reported that in midgestation (GD106–GD118) sheep fetuses, the HPG axis is active and inhibited by the testis but not the ovary (34, 35). Our results show that this regulatory system is active significantly earlier in gestation during the time when the oSDN is being masculinized by T (ie, GD60–GD85). The capacity for feedback regulation during the critical period maintains homeostasis and mitigates effects caused by variations in the circulating levels of T or perturbations in androgen action. Thus, we are the first to show that the brain masculinization program is actively defended in a long-gestation animal.
In agreement with previous reports (2, 7), we found that the volume of the oSDN in late-gestation fetal lambs was larger in males than in females. Previous studies also established that prenatal T masculinizes the volume of the oSDN and that blocking local estrogen synthesis in the fetal brain does not interfere with this process (2, 17). These results suggest that aromatization is not required for masculinization of the preoptic area in sheep in contrast to other animals, such as rodents (36). In the first experiment of this study, we found that maternal treatments from GD60 to GD90 with the androgen receptor antagonist flutamide significantly reduced the volume of the oSDN in GD135 males. As expected, flutamide had no effect in females, because in females, the oSDN develops independently of androgen control. Maternal injections of the nonaromatizable androgen receptor agonist DHT propionate also reduced the volume of oSDN in males and had little or no effect on the volume in females. The paradoxical result that androgen receptor activation reduced oSDN volume in males and failed to enlarge it in females led us to examine the regulation of the fetal HPG axis during the critical period of oSDN formation to determine whether the maternal treatments altered the hormonal environment of the fetus in a way that can explain these results.
Thus, in the second experiment, pregnant ewes were injected with vehicle, flutamide, or DHT propionate from GD60 to GD84, and plasma concentrations of DHT, T, E2, and LH were measured in fetuses delivered on GD85. The expression of the gonadotropin subunits in the anterior pituitary and GnRH and kisspeptin in the hypothalamus was also measured at this time to assess the site of hormone feedback action. CTL GD85 females exhibited significantly greater serum levels of LH compared with CTL males, whereas serum T levels were significantly higher in males than in females. The elevated levels of LH in females and the inverse relationship between serum T and LH in males confirms previous reports in sheep (37, 38) and indicates that the fetal testis, but not the ovary, feeds back to regulate LH secretion at this stage in development. The fetal testis has also been shown to play an important role in feedback regulation of LH secretion at midgestation in nonhuman primate (39) and human fetuses (40), although not in rodents (41).
Flutamide is considered a “pure” antiandrogen and requires hydroxylation in vivo to form 2-OH-flutamide, which competes with native T for binding to the androgen receptor. We found that 2-OH-flutamide crosses the placenta and reaches therapeutic concentrations in the fetus. Maternal injection of flutamide from GD60 to GD84 enhanced serum levels of LH and T and increased the weight of the testes on GD85. Flutamide had no effect on serum LH levels or ovarian weights in females. These results show that only male fetuses are subject to tonic negative feedback by androgen and that this occurs at least 3 weeks earlier than previously reported (20, 38). Antagonism of androgen action by flutamide in eugonadal males led to increased gonadotropin secretion, stimulation of the testis, and enhanced secretion of T. Considered together with the results of the first experiment, it is apparent that flutamide exerts sufficient androgen receptor antagonism to reduce oSDN volume even when serum T was elevated. In fact, the rise in serum T that accompanied this treatment in males may have partially counteracted flutamide's action and explain why the mean oSDN volume was intermediate between that of CTL males and CTL females. Alternatively, it is possible that androgen receptor antagonism increases T availability for aromatization, unmasking a potential role for E2 that was available in males but not females. This seems unlikely, because previous studies found that aromatase inhibition in the fetus fails to disrupt development of the oSDN in male fetuses (17). Future studies could further assess E2's effect on oSDN development by studying the effect of combined flutamide and exogenous T treatments on oSDN development in females, an approach that has been used to increase estrogen exposure and study the sexual differentiation of the timing of puberty in sheep (16). Another strategy would be to castrate male fetuses and treat directly with androgens and/or estrogens, although this is currently not feasible at this young gestational age.
In contrast to flutamide, maternal injection of DHT propionate slightly and significantly raised serum DHT levels in lamb fetuses. This was accompanied by the suppression of serum LH and reduction of testis weights in males. Serum T levels were also significantly reduced. In females, LH levels were suppressed, but ovary weight and serum E2 levels were unaffected. These results demonstrate that DHT exerts negative feedback on gonadotropin secretion and consequently reduces testicular T secretion in males, which can in turn explain why oSDN volume was reduced in males and unaffected in females. The low level of serum DHT achieved in fetuses after maternal treatment with DHT propionate was insufficient to masculinize the oSDN in females, yet it significantly inhibited the hypothalamic-pituitary axis and reduced serum LH and T levels enough to interfere with masculinization in males. By comparison, the total serum concentration of androgen (T + DHT) in males exposed to DHT was less than half the concentration in CTL males. Preliminary analysis performed by liquid chromatography mass spectroscopy indicates that there was significant conversion of DHT to 3α-androstanediol in fetal serum (our unpublished data). 3α-androstanediol is a weak androgen (Dissociation constant (Kd) = 10−6M for the androgen receptor) but can be converted back into DHT in the hypothalamus (29, 42, 43), suggesting that the primary steroid acting at the tissue level after exogenous administration of DHT propionate is the original androgen. It is also plausible that some effects of DHT could be caused by its conversion into the 3β-androstanediol, which is a high-affinity agonist of the estrogen receptor-β and does not bind to the androgen receptor (44). Estrogen receptor-β is expressed in midgestation lamb fetuses (45) and has been shown to modulate gonadotropin secretion in ovariectomized ewes (46). Thus, DHT may not act as a pure androgen in all tissues, and the possibility of its metabolism should be considered when interpreting the consequences of DHT exposure of oSDN development.
The finding that flutamide and DHT have opposite effects on serum levels of T helps explain the treatment effects on oSDN development. The response to flutamide demonstrates that androgen receptors mediate masculinization of oSDN in eugonadal males by directly blocking endogenous androgen action in the oSDN, so that although the T is significantly elevated, it is unable to overcome the flutamide blockade. The response to DHT supports this mechanism by showing that oSDN volume was reduced, because its negative feedback effect on LH suppressed serum T levels reducing androgen exposure to the developing oSDN.
The way in which sex steroids regulate gonadotropin synthesis is complex and can be mediated by effects on pulsatile GnRH secretion and/or direct effects at the level of the pituitary gonadotrophs (47, 48). We observed that steady state levels of FSHβ and α-subunit mRNA were significantly higher in CTL female than in male fetuses. In contrast, no sex difference in LHβ was observed. These data suggest that the testis suppresses FSH synthesis at least in part by acting on the pituitary, whereas LH negative feedback is exerted on GnRH. Although we did not measure serum FSH in the present study, previous studies showed that mean serum FSH levels are significantly higher in female than in male midgestation eugonadal lamb fetuses (37, 49). The sex difference in FSHβ mRNA suggests that inhibin produced by the testis may be involved in FSH regulation between GD60 and GD90. This is supported by measurement of plasma concentrations of inhibin, which are higher in male compared with female fetuses (35), and the observation that gonadectomy of sheep fetuses results in elevated FSH concentrations in males but not females (49).
Maternal injections of flutamide had no effect on gonadotropin subunit mRNAs in exposed fetuses, despite the observation that this treatment significantly elevated serum concentrations of LH in males. These results suggest that gonadotropin secretion can be uncoupled from gene transcription in eugonadal males. Similar results were obtained in adult male rats where flutamide treatment increases serum and pituitary LH and FSH levels without altering LHβ and FSHβ mRNA expression (50). In contrast, castration increases LHβ mRNA expression in a number of adult male mammals, including rats and sheep (47, 51). It is possible that the LH response to flutamide is mediated via the hypothalamus by regulating the secretion of GnRH into the hypophyseal portal circulation. Past studies demonstrating that LH secretion is pulsatile in the GD81 ovine fetus, controlled by GnRH (22–24, 34, 52, 53), and stimulated by N-methyl D-aspartate administration (54) strongly support the idea that pituitary gonadotropin secretion is not autonomous in the ovine fetus but actively regulated by hypothalamic GnRH. Moreover, the hypothalamus appears to be the site of negative feedback, because LH pulse frequency is significantly elevated after gonadectomy in GD108–GD130 male fetuses (38). The negative feedback effects of the fetal testis on LH secretion are most likely due to T, although this has never been directly tested by castration and replacement studies in sheep as it has in nonhuman primates (55).
Maternal treatments with DHT propionate decreased steady state LHβ and FSHβ mRNA levels but had no effect on α-subunit mRNA levels in exposed male and female fetuses. The effect on LHβ mRNA mirrored the effect on serum LH concentrations. It is not possible to conclude definitively from these results whether DHT decreases gonadotropin subunit synthesis directly by acting on the pituitary, indirectly by reducing GnRH secretion and gonadotrope stimulation or both. In adult rams, T and DHT act on the brain to suppress GnRH pulse frequency (56). In castrated hypothalamus-pituitary disconnected adult rams treated with GnRH pulses, treatments with T or DHT do not affect the amplitude of LH pulses or mean plasma concentrations (57). Despite this, there is evidence from other studies that a component of negative feedback by DHT occurs at the pituitary (58). Regardless of its site of action, it seems likely from our results that both T and its major androgenic metabolite DHT play important roles for the steroidal control of gonadotropin secretion in the ovine fetus. It remains to be determined whether T's estrogenic metabolite, E2, plays a role in the regulation of gonadotropin secretion in the lamb fetus as it appears to in adult rams (56, 59, 60).
We found that maternal injection of DHT propionate suppressed the expression of KISS1 mRNA in the MBH of exposed fetuses but had no effect on basal hypothalamic or MPOA GnRH mRNA expression. The effect of DHT on KISS1 expression mirrors its effect on LH secretion, suggesting that kisspeptin neurons in the hypothalamus relay the negative feedback effects of DHT in the ovine fetus. Interestingly, we also observed an overall main effect of sex, in which expression of both KISS1 and GnRH mRNA expression tended to be higher in females than in males. This may relate to the observation that during early midgestation, the hypothalamus and pituitary of males are suppressed by testicular T. Surprisingly, fetal KISS1 mRNA expression was not affected by maternal treatment with flutamide, although serum LH concentrations were elevated. One explanation could be that the elevated serum levels of T and E2 resulting from maternal flutamide treatment counteracted androgen receptor antagonism on kisspeptin synthesis. Alternatively, flutamide may act independent of transcriptional control to increase kisspeptin neural activity and secretion. Two major populations of kisspeptin neurons located in the anteroventral periventricular nucleus of the preoptic area and the arcuate nucleus in the MBH are found in rodents and sheep (61). Kisspeptin neurons in the arcuate are thought to relay T negative feedback to GnRH neurons, because castrated male sheep have higher numbers of kisspeptin-immunoreactive cells in arcuate, compared with age-matched intact CTLs (62), and T inhibits postcastration rise in KISS1 mRNA in the arcuate of male mice (63). Our results indicated that androgen receptors are involved in the regulation of KISS1 mRNA in the ovine fetus and provide evidence of a mechanism through which they can alter reproductive development and sexual differentiation in sheep.
In summary, our results provide convincing evidence that the prenatal program that masculinizes the oSDN in eugonadal male fetuses acts through the androgen receptor. We found that negative feedback regulation of the hypothalamus-pituitary-testis axis is active during the early midgestation critical period (ie, GD60–GD90) for oSDN differentiation. Perturbations that altered serum androgen levels or activity in male fetuses led to compensatory neuroendocrine adjustments that maintained the steroid endocrine milieu. These results can explain why both antagonist and agonist treatments reduced the oSDN in males without affecting females. The study also presents evidence that the hypothalamic kisspeptin system is a target for androgen action and may be involved in the regulation of fetal gonadotropin secretion. We postulate that the fetal hypothalamus-pituitary-testis axis in long gestation species such as sheep is sufficiently developed during the time of brain sexual differentiation to react to perturbations in serum androgen levels and defend against disruptions in brain masculinization.
Acknowledgments
We thank the Oregon State University students who cared for the sheep used in this study.
This work was supported by National Institutes of Health Grant R01OD011047 (to C.E.R.). Mass spectroscopy analysis performed by the Bioanalytical Shared Resource/Pharmacokinetic Core was supported by the Oregon Health and Science University Shared Resource Program. Hormonal analysis by the Endocrine Technology and Support Core was supported by the Oregon National Primate Research Center Core Grant P51 OD011092.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CTL
- control
- DHT
- dihydrotestosterone
- DMSO
- dimethyl sulfoxide
- E2
- estradiol
- GD
- gestational day
- HPG
- hypothalamus-pituitary-gonadal
- KISS1
- kisspeptin
- MBH
- medial basal hypothalamus
- MPOA
- medial preoptic area
- oSDN
- ovine sexually dimorphic nucleus
- T
- testosterone.
References
- 1. Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. [DOI] [PubMed] [Google Scholar]
- 2. Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. [DOI] [PubMed] [Google Scholar]
- 3. Ford JJ, D'Occhio MJ. Differentiation of sexual behavior in cattle, sheep and swine. J Anim Sci. 1989;67:1816–1823. [DOI] [PubMed] [Google Scholar]
- 4. Sweeney T, Saunders PT, Millar MR, Brooks AN. Ontogeny of anti-mullerian hormone, 3 β-hydroxysteroid dehydrogenase and androgen receptor expression during ovine fetal gonadal development. J Endocrinol. 1997;153:27–32. [DOI] [PubMed] [Google Scholar]
- 5. Quirke LD, Juengel JL, Tisdall DJ, Lun S, Heath DA, McNatty KP. Ontogeny of steroidogenesis in the fetal sheep gonad. Biol Reprod. 2001;65:216–228. [DOI] [PubMed] [Google Scholar]
- 6. Pomerantz DK, Nalbandov AV. Androgen level in the sheep fetus during gestation. Proc Soc Exp Biol Med. 1975;149:413–416. [DOI] [PubMed] [Google Scholar]
- 7. Roselli CE, Estill CT, Stadelman HL, Meaker M, Stormshak F. Separate critical periods exist for testosterone-induced differentiation of the brain and genitals in sheep. Endocrinology. 2011;152:2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roselli CE, Resko JA, Stormshak F. Estrogen synthesis in fetal sheep brain: effect of maternal treatment with an aromatase inhibitor. Biol Reprod. 2003;68:370–374. [DOI] [PubMed] [Google Scholar]
- 9. Celotti F, Negri-Cesi P, Poletti A. Steroid metabolism in the mammalian brain: 5α-reduction and aromatization. Brain Res Bull. 1997;44:365–375. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen PN, Billiards SS, Walker DW, Hirst JJ. Changes in 5α-pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep. Pediatr Res. 2003;53:956–964. [DOI] [PubMed] [Google Scholar]
- 11. Petratos S, Hirst JJ, Mendis S, Anikijenko P, Walker DW. Localization of P450scc and 5α-reductase type-2 in the cerebellum of fetal and newborn sheep. Dev Brain Res. 2000;123:81–86. [DOI] [PubMed] [Google Scholar]
- 12. Döhler KD, Coquelin A, Davis F, et al. Pre- and postnatal influence of an estrogen antagonist and an androgen antagonist on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Neuroendocrinology. 1986;42:443–448. [DOI] [PubMed] [Google Scholar]
- 13. Hines M, Alsum P, Roy M, Gorski RA, Goy RW. Estrogenic contributions to sexual differentiation in the female guinea pig: influences of diethylstilbestrol and tamoxifen on neural, behavioral, and ovarian development. Horm Behav. 1987;21:402–417. [DOI] [PubMed] [Google Scholar]
- 14. Baum MJ, Tobet SA, Cherry JA, Paredes RG. Estrogenic control of preoptic area development in a carnivore, the ferret. Cell Mol Neurobiol. 1996;16:117–128. [DOI] [PubMed] [Google Scholar]
- 15. Masek KS, Wood RI, Foster DL. Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology. 1999;140:3459–3466. [DOI] [PubMed] [Google Scholar]
- 16. Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roselli CE, Stormshak F. The neurobiology of sexual partner preferences in rams. Horm Behav. 2009;55:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine. 2006;29:501–511. [DOI] [PubMed] [Google Scholar]
- 19. Brooks AN, Currie IS, Gibson F, Thomas GB. Neuroendocrine regulation of sheep fetuses. J Reprod Fertil Suppl. 1992;45:69–84. [PubMed] [Google Scholar]
- 20. Mesiano S, Hart CS, Heyer BW, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus. XXVI. A sex difference in the effect of castration on the hypothalamic-pituitary gonadotropin unit in the ovine fetus. Endocrinology. 1991;129:3073–3079. [DOI] [PubMed] [Google Scholar]
- 21. Caldani M, Antoine M, Batailler M, Duittoz A. Ontogeny of GnRH systems. J Reprod Fertil Suppl. 1995;49:147–162. [PubMed] [Google Scholar]
- 22. Mueller PL, Sklar CA, Gluckman PD, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus. IX. Luteinizing hormone and follicle-stimulating hormone response to luteinizing hormone-releasing factor in mid- and late gestation and in the neonate. Endocrinology. 1981;108:881–886. [DOI] [PubMed] [Google Scholar]
- 23. Clark SJ, Ellis N, Styne DM, Gluckman PD, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus. XVII. Demonstration of pulsatile luteinizing hormone secretion by the fetal pituitary gland. Endocrinology. 1984;115:1774–1779. [DOI] [PubMed] [Google Scholar]
- 24. Thomas GB, McNeilly AS, Gibson F, Brooks AN. Effects of pituitary-gonadal suppression with a gonadotrophin-releasing hormone agonist on fetal gonadotrophin secretion, fetal gonadal development and maternal steroid secretion in the sheep. J Endocrinol. 1994;141:317–324. [DOI] [PubMed] [Google Scholar]
- 25. Roa J, Navarro VM, Tena-Sempere M. Kisspeptins in reproductive biology: consensus knowledge and recent developments. Biol Reprod. 2011;85:650–660. [DOI] [PubMed] [Google Scholar]
- 26. Smith JT. Sex steroid regulation of kisspeptin circuits. In: Kauffman AS, Smith JT, eds. Kisspeptin Signaling in Reproductive Biology. Advances in Experimental Biology and Medicine Vol 784 New York, NY: Springer; 2013:275–295. [DOI] [PubMed] [Google Scholar]
- 27. Murad F, Haynes RC. Androgens. In: Gilman AG, Goodman LS, Rall TW, Murad F, eds. The Pharmacological Basis of Therapeutics. New York, NY: MacMillan Publishing Co; 1985:1440–1458. [Google Scholar]
- 28. Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roselli CE, Stormshak F. Ontogeny of cytochrome P450 aromatase mRNA expression in the developing sheep brain. J Neuroendocrinol. 2012;24:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Resko JA, Ellinwood WE, Pasztor LM, Huhl AE. Sex steroids in the umbilical circulation of fetal rhesus monkeys from the time of gonadal differentiation. J Clin Endocrinol Metab. 1980;50:900–905. [DOI] [PubMed] [Google Scholar]
- 32. Niswender GD, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969;84:1166–1173. [DOI] [PubMed] [Google Scholar]
- 33. Zheng H, Wu D, Qian Z, Xiang Y. Determination of 2-hydroxyflutamide in human plasma by liquid chromatography-tandem mass spectrometry (LC-MS/MS): application to a bioequivalence study on Chinese volunteers. J Chromatogr B. 2010;878:1611–1615. [DOI] [PubMed] [Google Scholar]
- 34. Clark SJ, Hauffa BP, Rodens KP, Styne DL, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus: XIX: the effect of a potent luteinizing hormone-releasing factor agonist on gonadotropin and testosterone release in the fetus and neonate. Pediatr Res. 1989;25:347–352. [DOI] [PubMed] [Google Scholar]
- 35. Brooks AN, Hagan DM, Sheng C, McNeilly AS, Sweeney T. Prenatal gonadotrophins in the sheep. Anim Reprod Sci. 1996;42:471–481. [Google Scholar]
- 36. MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. [DOI] [PubMed] [Google Scholar]
- 37. Sklar CA, Mueller PL, Gluckman PD, Kaplan SL, Rudolph AM, Grumbach MM. Hormone ontogeny in the ovine fetus. VII. Circulating luteinizing hormone and follicle-stimulating hormone in mid- and late gestation. Endocrinology. 1981;108:874–880. [DOI] [PubMed] [Google Scholar]
- 38. Matwijiw I, Faiman C. Control of gonadotropin secretion in the ovine fetus. II. A sex difference in pulsatile luteinizing hormone secretion after castration. Endocrinology. 1989;124:1352–1358. [DOI] [PubMed] [Google Scholar]
- 39. Ellinwood WE, Baughman WL, Resko JA. Control of pituitary gonadotropin secretion in fetal rhesus macaques. In: Novy MJ, Resko JA, eds. Fetal Endocrinology. New York, NY: Academic Press; 1981:269–283. [Google Scholar]
- 40. Clements JA, Reyes FI, Winter JS, Faiman C. Studies on human sexual development. III. Fetal pituitary and serum, and amniotic fluid concentrations of LH, CG, and FSH. J Clin Endocrinol Metab. 1976;42:9–19. [DOI] [PubMed] [Google Scholar]
- 41. Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. [DOI] [PubMed] [Google Scholar]
- 42. Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279:10784–10795. [DOI] [PubMed] [Google Scholar]
- 43. Penning TM, Jin Y, Steckelbroeck S, Lanisnik Rizner T, Lewis M. Structure-function of human 3α-hydroxysteroid dehydrogenases: genes and proteins. Mol Cell Endocrinol. 2004;215:63–72. [DOI] [PubMed] [Google Scholar]
- 44. Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schaub CE, Gersting JA, Keller-Wood M, Wood CE. Development of ER-α and ER-β expression in the developing ovine brain and pituitary. Gene Expr Patterns. 2008;8:457–463. [DOI] [PubMed] [Google Scholar]
- 46. Arreguin-Arevalo JA, Davis TL, Nett TM. Differential modulation of gonadotropin secretion by selective estrogen receptor 1 and estrogen receptor 2 agonists in ovariectomized ewes. Biol Reprod. 2007;77:320–328. [DOI] [PubMed] [Google Scholar]
- 47. Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990;11:177–199. [DOI] [PubMed] [Google Scholar]
- 48. Mercer JE. Pituitary gonadotropin gene regulation. Mol Cell Endocrinol. 1990;73:C63–C67. [DOI] [PubMed] [Google Scholar]
- 49. Matwijiw I, Faiman C. Control of gonadotropin secretion in the ovine fetus. III. Effect of castration on serum follicle-stimulating hormone levels during the last trimester of gestation. Endocrinology. 1991;129:1443–1446. [DOI] [PubMed] [Google Scholar]
- 50. Gromoll J, Weinbauer GF, Simoni M, Nieschlag E. Effects of antiandrogens and ethane dimethane sulphonate (EDS) on gene expression, free subunits, bioactivity and secretion of pituitary gonadotrophins in male rats. Mol Cell Endocrinol. 1993;91:119–125. [DOI] [PubMed] [Google Scholar]
- 51. Pelletier J, Counis R, de Reviers MM, Moumni M, Tillet Y. Changes in LHβ-gene and FSHβ-gene expression in the ram pars tuberalis according to season and castration. Cell Tissue Res. 1995;281:127–133. [DOI] [PubMed] [Google Scholar]
- 52. Brooks AN, McNeilly AS. Inhibitory effects of a luteinizing-hormone-releasing hormone agonist implant on ovine fetal gonadotrophin secretion and pituitary sensitivity to luteinizing-hormone-releasing hormone. J Reprod Fertil. 1992;96:785–792. [DOI] [PubMed] [Google Scholar]
- 53. Matwijiw I, Faiman C. Control of gonadotropin secretion in the ovine fetus: the effects of a specific gonadotropin-releasing hormone antagonist on pulsatile luteinizing hormone secretion. Endocrinology. 1987;121:347–351. [DOI] [PubMed] [Google Scholar]
- 54. Bettendorf M, de Zegher F, Albers N, Hart CS, Kaplan SL, Grumbach MM. Acute N-methyl-D,L-aspartate administration stimulates the luteinizing hormone releasing hormone pulse generator in the ovine fetus. Horm Res. 1999;51:25–30. [DOI] [PubMed] [Google Scholar]
- 55. Resko JA, Ellinwood WE. Negative feedback regulation of gonadotropin secretion by androgens in fetal rhesus macaques. Biol Reprod. 1985;33:346–352. [DOI] [PubMed] [Google Scholar]
- 56. Tilbrook AJ, Clarke IJ. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol Reprod. 2001;64:735–742. [DOI] [PubMed] [Google Scholar]
- 57. Tilbrook AJ, de Kretser DM, Cummins JT, Clarke IJ. The negative feedback effects of testicular steroids are predominantly at the hypothalamus in the ram. Endocrinology. 1991;129:3080–3092. [DOI] [PubMed] [Google Scholar]
- 58. Schanbacher BD, Johnson MP, Tindall DJ. Androgenic regulation of luteinizing hormone secretion: relationship to androgen binding in sheep pituitary. Biol Reprod. 1987;36:340–350. [DOI] [PubMed] [Google Scholar]
- 59. Sharma TP, Blache D, Blackberry MA, Martin GB. Role of peripheral and central aromatization in the control of gonadotrophin secretion in the male sheep. Reprod Fertil Dev. 1999;11:293–302. [DOI] [PubMed] [Google Scholar]
- 60. D'Occhio MJ, Schanbacher BD, Kinder JE. Androgenic and oestrogenic steroid participation in feedback control of luteinizing hormone secretion in male sheep. Acta Endocrinol. 1983;102:499–504. [DOI] [PubMed] [Google Scholar]
- 61. Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides. 2009;30:94–102. [DOI] [PubMed] [Google Scholar]
- 62. Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153:2756–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. [DOI] [PubMed] [Google Scholar]