Abstract
The maturation of the intestine into the adult form involves the formation of adult stem cells in a thyroid hormone (T3)-dependent process in vertebrates. In mammals, this takes place during postembryonic development, a period around birth when the T3 level peaks. Due to the difficulty of manipulating late-stage, uterus-enclosed embryos, very little is known about the development of the adult intestinal stem cells. Interestingly, the remodeling of the intestine during the T3-dependent amphibian metamorphosis mimics the maturation of mammalian intestine. Our earlier microarray studies in Xenopus laevis revealed that the transcription factor SRY (sex-determining region Y)-box 3 (Sox3), well known for its involvement in neural development, was upregulated in the intestinal epithelium during metamorphosis. Here, we show that Sox3 is highly and specifically expressed in the developing adult intestinal progenitor/stem cells. We further show that its induction by T3 is independent of new protein synthesis, suggesting that Sox3 is directly activated by liganded T3 receptor. Thus, T3 activates Sox3 as one of the earliest changes in the epithelium, and Sox3 in turn may facilitate the dedifferentiation of the larval epithelial cells into adult stem cells.
Thyroid hormone (T3) regulates the homeostasis and development of diverse organs in vertebrates. Perhaps the most critical period that requires T3 during mammalian development is the so-called postembryonic development, which is around birth when the T3 level peaks. Many organs mature into the adult form during this developmental process. One such organ is the intestine, whose maturation appears to involve the formation of adult epithelial stem cells (1–4).
The intestine has been used extensively as a model organ for studying adult stem cell (ASC) function due to the continuous self-renewal of the epithelium (Ep) throughout adult life in vertebrates (1, 2, 5–9). In mammals, the epithelial self-renewal is achieved via the proliferation of stem cells residing near the base of each intestinal crypt, followed by their differentiation as they move along the crypt-villus axis. After a finite period of time, the differentiated epithelial cells are removed via apoptosis at the tip of the villus. Extensive studies have led to the identification of a number of signaling pathways important for intestinal development and adult cell renewal during intestinal homeostasis and neoplasia development (7, 10). On the other hand, little is known about how the ASCs are formed during development, largely due to the difficulty of manipulating late-stage, uterus-enclosed mammalian embryos.
Amphibian metamorphosis resembles postembryonic development in mammals, including intestinal maturation and dependence on T3 (11–14). Unlike postembryonic development in mammals, this process can be easily manipulated in intact animals in vivo or even in organ or primary cell cultures by controlling the availability of T3 (11, 12, 15, 16). This has made amphibian metamorphosis an excellent model to study adult organ development in vertebrates.
During metamorphosis, the intestine is remodeled into the adult form similar to that in adult mammals. Furthermore, both intestinal metamorphosis and the formation of mammalian adult intestine are dependent on T3 and involve similar processes (1–4, 17–21). Thus, we have been studying intestinal metamorphosis in Xenopus laevis to understand the formation of ASCs during vertebrate development. During metamorphosis, the simple tubular larval intestine consisting of predominantly a single layer of primary Ep is transformed into a complex adult structure with a multiply folded Ep (22). In the Ep, the larval epithelial cells undergo degeneration through programmed cell death or apoptosis, and adult intestinal stem cells are formed de novo in a T3-dependent process (1, 13, 22–25).
T3 is known to affect metamorphosis by regulating gene transcription through T3 receptors (TRs) (26–30). Toward identifying the molecular basis underlying the formation of the stem cells, we have previously carried out a microarray analysis on isolated intestinal Ep and the rest of the intestine, or the non-Ep, at different stages of X. laevis metamorphosis. This led to the identifications of many genes that are regulated by T3 in Ep, non-Ep, or both (31). Our earlier work has shown that T3 action in the Ep alone was sufficient to induce the dedifferentiation of some larval epithelial cells to become precursor of ASCs, although the formation of the stem cells also requires T3 action in the non-Ep (23, 25, 32). Thus, one or more of the genes regulated by T3 in the Ep play a critical role in the early stages of ASC formation. Among the genes that were found to be highly expressed in the Ep at the climax of metamorphosis when most of the cells in the Ep were proliferating ASCs (14, 22) is a X. laevis homolog of the mammalian Sox3 gene. The mammalian Sox3 gene belongs to the large family of transcription factors that were first identified based on homology to the HMG box in the gene for the mammalian testis determining factor SRY (sex-determining region Y) (33–35). Sox3 belongs to the SoxB1 subfamily that also include Sox1, Sox2, and Sox3 (35), all of which have been implicated in vertebrate neurogenesis during development (35–49). We show here that Sox3 (SRY [sex-determining region Y]-box 3) is directly activated by T3 in the Ep but not in the non-Ep and that its expression correlates with ASC development and/or proliferation in the metamorphosing intestine, suggesting that Sox3 plays a role early in larval epithelial cell dedifferentiation toward adult intestinal stem cells.
Materials and Methods
Experimental animals
X. laevis tadpoles were purchased from NASCO or produced in the laboratory, and staged according to Ref. 50. All animals were maintained and used as approved by the Animal Use and Care Committee of Eunice Kennedy Shriver National Institutes of Child Health and Human Development, National Institutes of Health.
Premetamorphic X. laevis tadpoles at stage 54 were treated with 100nM T3 and/or protein synthesis inhibitors cycloheximide (20 μg/mL) and anisomycin (25 μg/mL) as described (51) or with 5nM T3 for 0 to 7 days. RNA was isolated from tissues/organs for gene expression analysis.
RNA isolation
Total RNA isolation from whole animals was done as described before (52, 53). Intestines were dissected from X. laevis tadpoles at stages 54 to 66 or tadpoles at stage 54 after the indicated treatment and used for RNA isolation as described (54). Intestinal tissue-specific RNA was isolated from stage 56, 61, and 66 tadpoles as reported (31). Total RNA isolation was done with TRIzol reagent (Invitrogen) and made DNA-free with ribonuclease-free deoxyribonuclease I (Ambion) treatment.
Quantitative RT-PCR
One microgram of total RNA was used to synthesize cDNA with the High-Capacity cDNA reverse transcription kit (Applied Biosystems Inc.) following the manufacturer's instructions. Sox3 expression or internal control gene expression in each sample was determined by quantitative PCR analysis with SYBR-Green dye (Applied Biosystems Inc). The primers were 5′-GCAATAAAGCCAGTCAGGACCG-3′ (forward) and 5′-TCTCTTCTCAGCCTCCGACATAAC-3′ (reverse) for Sox1, 5′-CTCCAACTCTGCGTCCAACAAC-3′ (forward) and 5′-TCTCCTGAGCCATCTTTCTCCTC-3′ (reverse) for Sox2, and 5′-CGATGAACGCGTTTATGGTTT-3′ (forward) and 5′-TACTTGTAATCCGGGTAGTC-3′ (reverse) for Sox3. A set of primers specific for ribosomal protein rpl8 (55) or EF1A (31, 51) was used as the control for RNA input for each sample, and the Sox3 expression level in each sample was normalized to that of rpl8 or EF1A. At least 3 animals were pooled together for each sample. Quantitative RT-PCR (qRT-PCR) were done in triplicates and the mean and SE are presented. Statistical analysis was done through ANOVA with Tukey's multiple-component test. Each experiment was repeated at least twice with similar results.
In situ hybridization
A 963-bp fragment encompassing the 5′-untranslated region (UTR) (86 bp) and the coding region (877 bp) of Sox3 cDNA was PCR amplified by using the primers 5′-GGGAGTTACAGGGAAGTTTTGTGC-3′ (forward) and 5′-CACTCTGGTAGTGTTGGTGTACACTA-3′ (reverse) and a 919-bp fragment encompassing the coding region (338 bp) and the 3′-UTR (581 bp) of Sox3 cDNA was PCR amplified by using the following primers: 5′-GCCCCATGATGTCCTCTGCGCAGAC-3′ (forward) and 5′-TTACATTCCTCATAGAAAAATTTATTA-3′ (reverse). Both PCR products were subcloned into pCR-Blunt II-TOPO cloning vector (Invitrogen) and sequenced. The resulting plasmid containing the 5′-UTR and coding region was linearized with BamHI to synthesize an antisense probe with T7 RNA polymerase, and the plasmid containing the coding region and 3′-UTR was linearized with NotI to synthesize an antisense probe with SP6 RNA polymerase, respectively, both by using a digoxigenin RNA labeling kit (Roche Applied Science). This produced 2 in situ probes that together covered nearly the full length of the Sox3 mRNA. Both probes were used together to enhance the in situ hybridization signal. The in situ probe for LGR5 was made essentially as previous described (54). Intestinal fragments were isolated from the anterior part of the small intestine after the bile duct junction from tadpoles at indicated stages and fixed in 1×MEMFA (100 mM MOPS [pH 7.4], 2 mM EGTA, 1 mM MgSO4, 3.7% [v/v] formaldehyde), followed by cryosectioning. Tissue sections cut at 10 μm were subjected to in situ hybridization by using a mixture of both antisense probes as previously described (54). Photographs were taken by using a digital CCD color camera (Retiga Exi; QImaging) attached to an optical microscope (BX60; Olympus).
Results
Xenopus Sox3 is highly expressed in the intestinal Ep at the climax of metamorphosis
The SoxB1 subfamily includes Sox1, Sox2, and Sox3 genes. Frog SoxB1 genes were identified through a BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) by using conserved mammalian SoxB1 protein sequences. Sequence analyses revealed that both X. laevis and the highly related species Xenopus tropicalis have all 3 SoxB1 genes, and there are 2 Sox3 genes in X. laevis due to the duplication of most of the genome in this pseudotetraploid species (Figure 1).
Figure 1.

Phyolgenetic analysis of Xenopus SoxB1 genes. The SoxB1 subfamily of Sox genes, Sox1, Sox2, and Sox3 from X. laevis (Xl), X. tropicalis (Xt), Rana rogusa (Rr), and human (h) were analyzed by using MegAlign (DNASTAR). Note that there are 2 Sox3 genes in the pseudotetraploid X. laevis due to gene duplication.
To investigate the involvement of SoxB1 genes during metamorphosis, we first analyzed the expression of the SoxB1 genes in whole animals from embryonic stage 30 to the end of metamorphosis (stage 66). As shown in Figure 2, Sox1 and Sox2 had very similar expression profiles, with relatively high levels of expression during embryogenesis (30–35) and in feeding tadpoles (stage 45). Their expression was at much lower but stable levels during metamorphosis (stage 54–66). On the other hand, Sox3 was expressed at low levels during embryogenesis, and its expression increased to high levels by feeding stage (stage 45) (Figure 2). Interestingly, its expression was gradually repressed during metamorphosis (Figure 2), suggesting that Sox3 gene expression is no longer needed during the late stage of metamorphosis.
Figure 2.
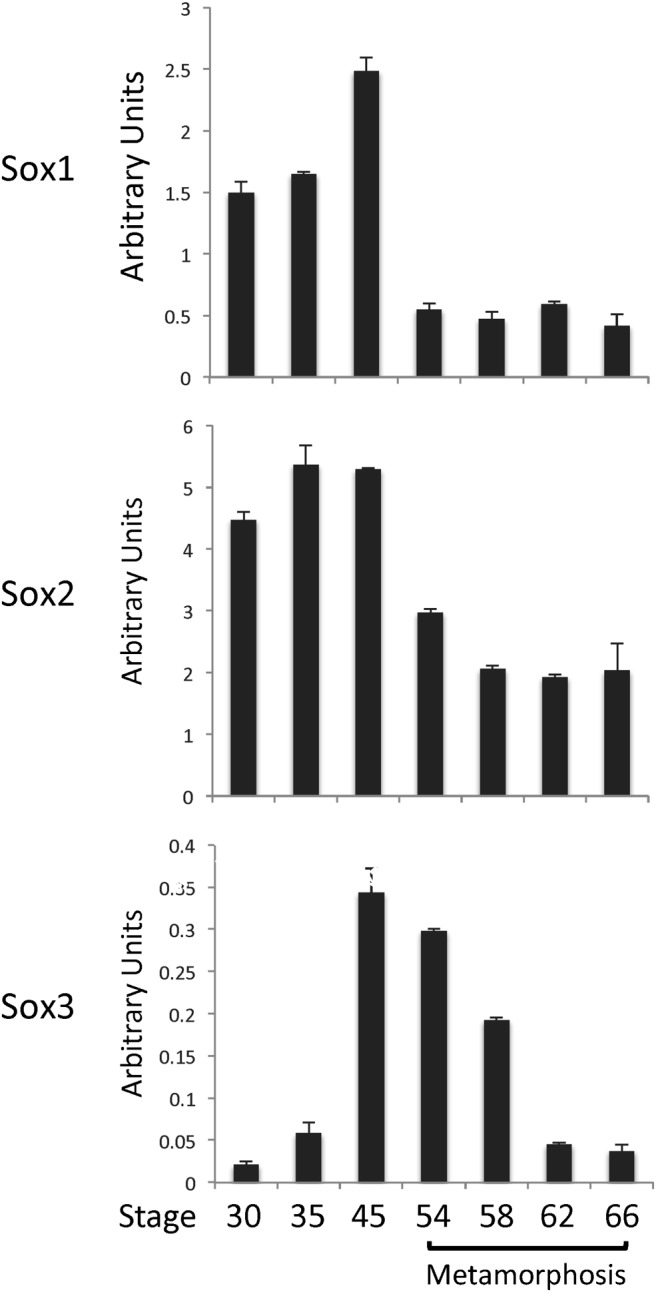
Distinct expression patterns of SoxB1 genes during X. laevis development. Total RNA was extracted from whole animals at the indicated stages and subjected to qRT-PCR analysis of SoxB1 gene expression. The expression of SoxB1 genes was normalized to that of EF1A. The mean of triplicates is presented with the SE. Note that only Sox3 was regulated during metamorphosis.
Our earlier microarray study identified Sox3 but not Sox1 or Sox2 as a gene upregulated during intestinal metamorphosis (31). This and the observation in the whole animals above prompted us to investigate possible involvement of Sox3 during intestinal metamorphosis. By using a pair of primers recognizing both Sox3A and -B in X. laevis for RT-PCR analysis, we found no detectable Sox3 mRNA at premetamorphic stages (stages 54–56) or at the end of metamorphosis (stage 66) (Figure 3A). Sox3 expression was activated by stage 58, the early metamorphic climax when the plasma T3 level was near half of the maximum during development (56). The expression reached peak levels at stage 62, when most of the epithelial cells are proliferating ASCs with few larval epithelial cells undergoing apoptosis (14, 22). Thus, Sox3 mRNA levels temporally correlate with the development and/or proliferation of the adult epithelial stem cells.
Figure 3.
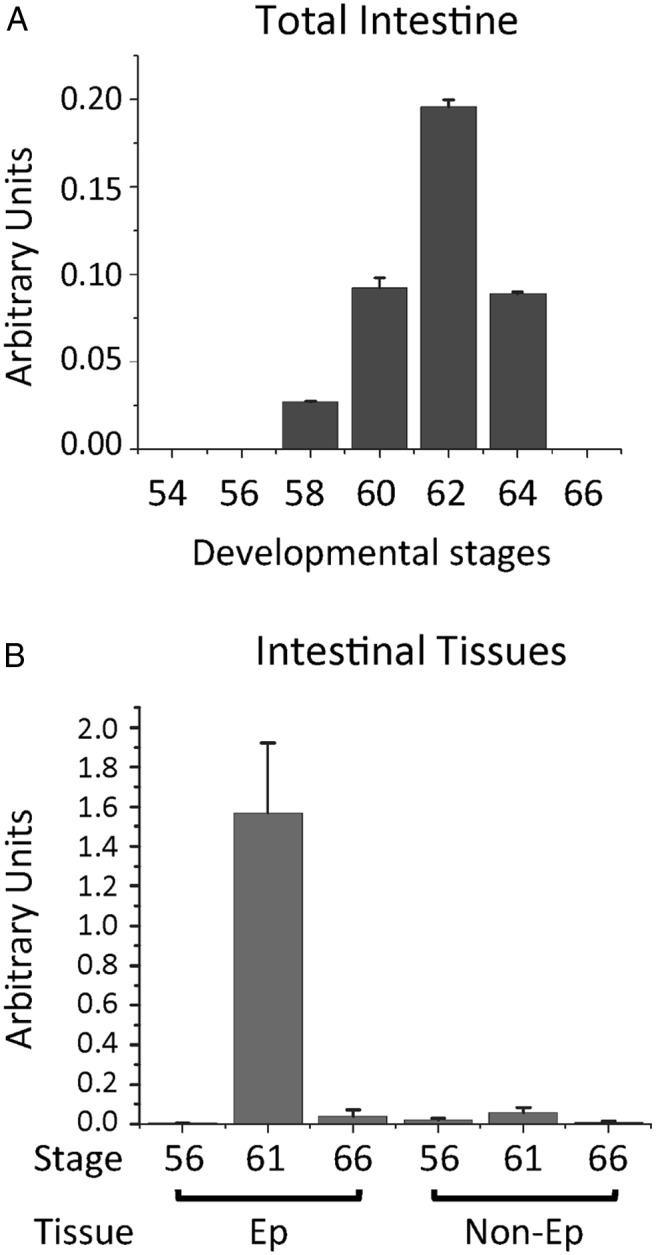
Tissue-specific developmental regulation of Sox3 in intestine during X. laevis metamorphosis. A, Sox3 is highly expressed only during metamorphosis. Total intestinal RNA at different stages was analyzed by qRT-PCR. Note that little Sox3 expression was found either before (stages 54–56) or at the end (stage 66) of metamorphosis. B, Sox3 is expressed only in the intestinal Ep. Total RNA was isolated from Ep and non-Ep of the intestine at different stages of development, 56 (premetamorphosis), 61 (climax), and 66 (end of metamorphosis), and analyzed by qRT-PCR. Note that Sox3 was highly expressed only in the Ep at the climax of metamorphosis when stem cells were forming or proliferating. The expression level was normalized to the control gene and shown in arbitrary units, and the mean of triplicates is presented with the SE.
Next, we analyzed the expression of Sox3 in the Ep and non-Ep isolated from the intestine at premetamorphic stage 56, climax (stage 61), and end of metamorphosis (stage 66). Consistent with the microarray findings (31), Sox3 was found to be expressed almost exclusively in the Ep at the climax of metamorphosis (Figure 3B). Little mRNA was observed even in the Ep before or at the end of metamorphosis, indicating that Sox3 is uniquely expressed during the transformation of the intestinal Ep during metamorphosis.
Sox3 is induced by T3 treatment of premetamorphic tadpoles
T3 is the causative agent of metamorphosis and functions by regulating gene transcription through TRs (26–29). The expression profile of Sox3 during development suggests that Sox3 is regulated by T3. To investigate this possibility, we treated premetamorphic tadpoles, when there is little endogenous T3, with physiological levels of T3 (5nM) for 0 to 7 days. The qRT-PCR analysis revealed that Sox3 mRNA level was upregulated within 1 day of T3 treatment and reached a peak by 3 days before dropping off to low levels by 7 days (Figure 4A), mimicking the developmental profile (Figure 3A). Thus, Sox3 regulation during both natural and T3-induced metamorphosis correlates with intestinal stem cell development and/or proliferation.
Figure 4.
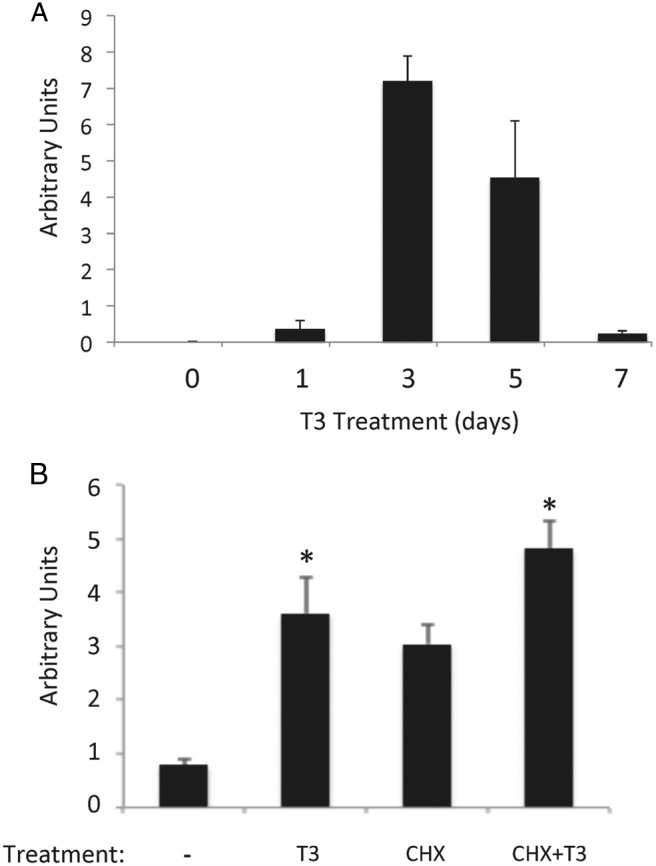
Sox3 is a target gene of T3. A, Sox3 is strongly induced by T3 treatment of premetamorphic tadpoles. X. laevis tadpoles at premetamorphic stage 54 were treated with T3 for 0 to 7 days, and total intestinal RNA was isolated and subjected to qRT-PCR analysis as in Figure 3. Note that Sox3 expression was strongly activated as early as 1 day after treatment and peaked around 3 days. B, T3 induces Sox3 expression independently of new protein synthesis. X. laevis tadpoles at premetamorphic stage 54 were treated with T3 in the presence or absence of protein synthesis inhibitors (cycloheximide and anisomycin [CHX]). Total RNA was isolated from the intestine and subjected to qRT-PCR analysis as in Figure 3. Note that T3 induced the expression of Sox3 even in the presence of protein synthesis inhibitors. The mean of triplicates is presented with the SE. The significant difference among different treatments was analyzed by ANOVA with Tukey's multiple-component test. *, P < .05.
T3 activates Sox3 gene in the absence of new protein synthesis
The strong induction of Sox3 expression by T3 after 1 day of treatment in premetamorphic tadpoles raised the possibility that Sox3 is an immediate early target gene of T3. To investigate this, we treated premetamorphic tadpoles with T3 in the presence or absence of protein synthesis inhibitors to block new protein synthesis (51, 57). Total intestinal RNA was isolated and analyzed for Sox3 expression. As shown in Figure 4B, Sox3 expression was upregulated by T3 both in the presence and absence of protein synthesis inhibitors, although protein synthesis inhibitors also stabilized Sox3 mRNA, leading to an increased level. (Note that the magnitude of T3 induction of Sox3 expression was less in the presence of protein synthesis inhibitors compared with that in the absence of protein synthesis inhibitors. This likely suggests that continued protein synthesis is required to maintain the level of one or more proteins important for Sox3 expression and/or that one or more proteins induced by T3 may help further increase the expression of Sox3.) Thus, Sox3 upregulation by T3 is one of the earliest events in T3-induced epithelial transformation.
Sox3 is expressed in the developing/proliferating ASCs
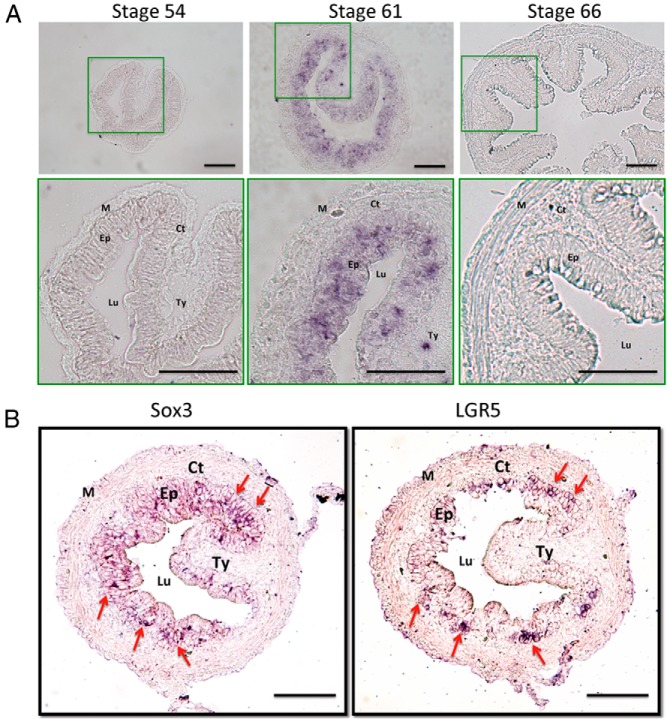
The exclusive expression of Sox3 in the Ep at the climax of intestinal metamorphosis argues that Sox3 is expressed in the proliferating adult progenitor/stem cells. To determine the cells expressing Sox3 in the intestine, in situ hybridization was carried out. Consistent with the RT-PCR analyses, we found little signal in the intestine in premetamorphic (stage 54) or postmetamorphic (stage 66) tadpoles (Figure 5A). On the other hand, strong signals were observed exclusively in the Ep at the climax (stage 61) of metamorphosis (Figure 5A). We and others have previously shown that at the climax of metamorphosis, the Ep consists of essentially all adult progenitor/stem cells with few remaining larval epithelial cells undergoing apoptosis (22, 58–60). Indeed, by analyzing the expression of Sox3 and a well-known intestinal stem cell marker LGR5 on serial sections of stage 62 intestines, we observed that Sox3-expressing cells largely overlapped with those cells expressing LGR5 (Figure 5B). Thus, both the tissue-specific RT-PCR and in situ hybridization results support specific expression of Sox3 in the adult progenitor/stem cells.
Figure 5.
Sox3 is expressed in the proliferating adult epithelial cells at the climax of intestinal remodeling. A, Intestinal sections of X. laevis tadpoles at the premetamorphic stage (stage 54), the climax (stage 61), or the end (stage 66) of metamorphosis were analyzed by in situ hybridization with digoxigenin-labeled Sox3 RNA probes and visualized with AP-linked antidigoxigenin antibody and BCIP/NBT staining. B, Serial sections of the intestine of X. laevis tadpoles at the climax (stage 62) were analyzed by in situ hybridization with digoxigenin-labeled Sox3 RNA or LGR5 probes, respectively, and visualized with AP-linked anti-digoxigenin antibody and BCIP/NBT staining. Note that Sox3 expressing cells largely overlapped with those ones expressing LGR5. Arrows indicate cell clusters expressing both Sox3 and LGR5. Abbreviations: Ct, connective tissue; Lu, lumen; M, muscle; Ty, typhlosole. Scale bar, 100 μm.
Discussion
Increasing evidence has shown that T3 can affect stem cell development and function, especially during postembryonic development when T3 levels peak in vertebrates (9, 11, 13, 17–20, 61–64). Given the importance of adult organ-specific stem cells in organ homeostasis, understanding the formation of such stem cells is critical for the potential use of stem cells in tissue repair and regeneration. The formation of the adult intestine during frog metamorphosis mimics the maturation of the mouse intestine around birth (2–4, 11, 13, 62, 63) and thus serves as an excellent model for studying the molecular basis of stem cell formation. Here, we have provided evidence to suggest that the transcription factor Sox3, previously known to be involved in neurodevelopment, is induced by T3 in the Ep to facilitate the larval epithelial cell dedifferentiation into ASCs.
Due to their importance for organ homeostasis and tissue repair and regeneration, adult organ-specific stem cells, especially in the intestine, have been studied extensively in recent years. However, to date, little is known about the molecular mechanism governing the formation of such ASCs in vertebrates. Using intestinal remodeling during X. laevis metamorphosis as a model, we have previously shown that T3 induces tissue autonomous dedifferentiation of some larval epithelial cells to express sonic hedgehog (25), a gene known to be expressed in the developing adult epithelial stem cells (65–68), although the formation of ASCs, ie, cells expressing 2 well-known markers of the adult intestinal stem cells in mammals, also requires T3 induction in the non-Ep (25). Thus, T3-induced changes in both the Ep and the rest of the intestine, the non-Ep, are required for the development of adult intestinal stem cells during amphibian metamorphosis (13, 25). In an effort to identify such T3-induced changes, we previously carried out a gene expression microarray analysis on isolated Ep and non-Ep of X. laevis intestine from different metamorphic stages (31). This led to the discovery of many genes highly expressed in the Ep at the climax of metamorphosis. Among them is the gene that encodes the X. laevis homolog of the mammalian Sox3 gene.
Our analyses here showed that Sox3 was induced by T3 quickly and independently of new protein synthesis, suggesting that T3 directly activates the Sox3 promoter. A bioinformatics search of an approximately 10-kb available sequence of the promoter region revealed a putative thyroid hormone response element (TRE) about 3.5 kb upstream of the 5′-end of the reported Xenopus Sox3B mRNA that is capable of binding to TR in vitro (data not shown). However, we failed to detect any in vivo TR binding to the TRE region in the intestine by chromatin immunoprecipitation assay (data not shown). It is possible that the TR binding to the Sox3 promoter region is very weak and thus difficult to be detected by the chromatin immunoprecipitation assay. This may be consistent with the fact that the induction of Sox3 gene by T3 was weak after 1 day but much stronger after 3 days. Such T3-induction kinetics suggests that the initial activation via TR may be weak and the subsequent strong activation requires the synthesis of other transcription factors induced by T3 and is through a mechanism not involving direct TR binding to the promoter region. Alternatively, in the absence of complete genome sequence information, we cannot rule out the possibility that the functional TRE lies further upstream or downstream of the transcription start site. Clearly, further studies are needed to determine the exact mechanism of Sox3 activation by T3.
Sox3 belongs to the SoxB1 subfamily of the Sox gene family. This subfamily also includes Sox1 and Sox2 and all 3 SoxB1 genes are conserved among vertebrates. Earlier studies have shown that all SoxB1 genes play roles in inducing neural progenitor cell formation during early embryonic development in vertebrates and are required for stem-cell maintenance in the central nervous system (36–49). In particular, Sox3 has been associated with hypopituitarism and mental retardation, and a mouse conditional knockout study has shown that Sox3 is required for the formation of hypothalamus-pituitary axis, whereas Sox3 total knockout animals fail to develop due to gastrulation defect (40). Thus, the roles of Sox3 in later development and in most organs/tissues remain to be investigated. Our analyses here suggest that Sox3 may be important for the formation of adult organ-specific stem cells at late embryonic stages, during the so-called postembryonic period when adult organs develop/mature in vertebrates.
It is of interest to note that studies on induced pluripotent stem cells have clearly demonstrated the critical importance of transcription factors in determining stem cell identity (69, 70). In particular, 1 of the 4 transcription factors that were initially found to be sufficient to reprogram fibroblasts into pluripotent stem cells is Sox2 (70), a member of the SoxB1 subfamily. Thus, SoxB1 family members likely participate in the formation of ASCs in various organs/tissues during vertebrate development. Interestingly, in the central nervous system, Sox2 and Sox3 (as well as Sox1) are required for stem-cell maintenance (37). Their functional similarity is likely due to the highly conserved nature of the DNA-binding HMG domain among the 3 SoxB1 genes, which allows the regulation of many common genes by the Sox1, Sox2, and Sox3 members (35). On the other hand, the rest of the Sox2 and Sox3 proteins are much more divergent (35), suggesting possible different effects on the target genes. Thus, both Sox2 and Sox3 are likely involved in the formation of adult organ-specific stem cells as well as the maintenance of such stem cells, although the exact function is likely organ/tissue-dependent. Clearly, future studies by using approaches such as gain-of-function via transgenesis (71) or loss-of-function via knockdown (72) are needed to determine the exact role of Sox genes in ASCs during Xenopus development.
Whether and how Sox3 regulates the formation and/or proliferation of the ASCs remain to be investigated. As a transcription factor, it likely regulates the transcription of downstream genes important for stem cell development. In addition, it has been shown that several members of the Sox family, including Sox3, can directly bind to β-catenin in frogs and mice, thereby reducing Wnt signaling (73, 74), which is known to be important for adult intestinal stem cells (7, 10, 75). Thus, Sox3 may also function through interactions with Wnt or other signaling pathways. Identifying Sox3 target genes and the mechanisms of their regulation by Sox3 will be essential to understand how Sox3 participates in the development of adult organ-specific stem cells.
Acknowledgments
This work was supported by the intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and National Natural Science Foundation of China (Grants 31370187 and 30870113).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ASC
- adult stem cell
- qRT-PCR
- quantitative RT-PCR
- Sox3
- SRY (sex-determining region Y)-box 3
- T3
- thyroid hormone
- TR
- T3 receptor
- TRE
- thyroid hormone response element
- UTR
- untranslated region.
References
- 1. Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. The development of the adult intestinal stem cells: Insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci. 2011;1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ishizuya-Oka A, Shi YB. Evolutionary insights into postembryonic development of adult intestinal stem cells. Cell Biosci. 2011;1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muncan V, Heijmans J, Krasinski SD, et al. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun. 2011;2:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci U S A. 2011;108:10585–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacDonald WC, Trier JS, Everett NB. Cell proliferation and migration in the stomach, duodenum, and rectum of man: radioautographic studies. Gastroenterology. 1964;46:405–417. [PubMed] [Google Scholar]
- 6. Toner PG, Carr KE, Wyburn GM. The Digestive System: An Ultrastructural Atlas and Review. London, UK: Butterworth; 1971. [Google Scholar]
- 7. van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. [DOI] [PubMed] [Google Scholar]
- 8. Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974;141:503–519. [DOI] [PubMed] [Google Scholar]
- 9. Sun G, Shi YB. Thyroid hormone regulation of adult intestinal stem cell development: mechanisms and evolutionary conservations. Int J Biol Sci. 2012;8:1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. [DOI] [PubMed] [Google Scholar]
- 11. Tata JR. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays. 1993;15:239–248. [DOI] [PubMed] [Google Scholar]
- 12. Shi YB. Amphibian Metamorphosis: From Morphology to Molecular Biology. New York, NY: John Wiley, Sons; 1999. [Google Scholar]
- 13. Hasebe T, Fu L, Miller TC, Zhang Y, Shi YB, Ishizuya-Oka A. Thyroid hormone-induced cell-cell interactions are required for the development of adult intestinal stem cells. Cell Biosci. 2013;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi YB, Ishizuya-Oka A. Thyroid hormone regulation of apoptotic tissue remodeling: Implications from molecular analysis of amphibian metamorphosis. Prog Nucleic Acid Res Mol Biol. 2001;65:53–100. [DOI] [PubMed] [Google Scholar]
- 15. Su Y, Shi Y, Shi YB. Cyclosporin A but not FK506 inhibits thyroid hormone-induced apoptosis in tadpole intestinal epithelium. FASEB J. 1997;11:559–565. [DOI] [PubMed] [Google Scholar]
- 16. Su Y, Shi Y, Stolow MA, Shi YB. Thyroid hormone induces apoptosis in primary cell cultures of tadpole intestine: cell type specificity and effects of extracellular matrix. J Cell Biol. 1997;139:1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plateroti M, Gauthier K, Domon-Dell C, Freund JN, Samarut J, Chassande O. Functional interference between thyroid hormone receptor alpha (TRalpha) and natural truncated TRDeltaalpha isoforms in the control of intestine development. Mol Cell Biol. 2001;21:4761–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flamant F, Poguet AL, Plateroti M, et al. Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRalpha gene. Mol Endocrinol. 2002;16:24–32. [DOI] [PubMed] [Google Scholar]
- 19. Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J Biol Chem. 2009;284:1234–1241. [DOI] [PubMed] [Google Scholar]
- 20. Plateroti M, Chassande O, Fraichard A, et al. Involvement of T3Ralpha- and beta-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology. 1999;116:1367–1378. [DOI] [PubMed] [Google Scholar]
- 21. Heimeier RA, Das B, Buchholz DR, Fiorentino M, Shi YB. Studies on Xenopus laevis intestine reveal biological pathways underlying vertebrate gut adaptation from embryo to adult. Genome Biol. 2010;11:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi YB, Ishizuya-Oka A. Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr Top Dev Biol. 1996;32:205–235. [DOI] [PubMed] [Google Scholar]
- 23. Ishizuya-Oka A, Shimozawa A. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Roux's Arch Dev Biol. 1992;201:322–329. [DOI] [PubMed] [Google Scholar]
- 24. Ishizuya-Oka A, Shimozawa A. Inductive action of epithelium on differentiation of intestinal connective tissue of Xenopus laevis tadpoles during metamorphosis in vitro. Cell Tissue Res. 1994;277:427–436. [DOI] [PubMed] [Google Scholar]
- 25. Hasebe T, Buchholz DR, Shi YB, Ishizuya-Oka A. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells. 2011;29:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buchholz DR, Tomita A, Fu L, Paul BD, Shi YB. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol. 2004;24:9026–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buchholz DR, Hsia SC, Fu L, Shi YB. A dominant-negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol. 2003;23:6750–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci U S A. 2001;98:10739–10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi YB. Dual functions of thyroid hormone receptors in vertebrate development: the roles of histone-modifying cofactor complexes. Thyroid. 2009;19:987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi YB, Matsuura K, Fujimoto K, Wen L, Fu L. Thyroid hormone receptor actions on transcription in amphibia: the roles of histone modification and chromatin disruption. Cell Biosci. 2012;2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun G, Heimeier RA, Fu L, et al. Expression profiling of intestinal tissues implicates tissue-specific genes and pathways essential for thyroid hormone-induced adult stem cell development. Endocrinology. 2013;154:4396–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schreiber AM, Mukhi S, Brown DD. Cell-cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol. 2009;331:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lovell-Badge R. The early history of the Sox genes. Int J Biochem Cell Biol. 2010;42:378–380. [DOI] [PubMed] [Google Scholar]
- 34. Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. [DOI] [PubMed] [Google Scholar]
- 35. Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000;227:239–255. [DOI] [PubMed] [Google Scholar]
- 36. Archer TC, Jin J, Casey ES. Interaction of Sox1, Sox2, Sox3 and Oct4 during primary neurogenesis. Dev Biol. 2011;350:429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28:583–588. [DOI] [PubMed] [Google Scholar]
- 38. Kelberman D, de Castro SC, Huang S, et al. SOX2 plays a critical role in the pituitary, forebrain, and eye during human embryonic development. J Clin Endocrinol Metab. 2008;93:1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A. 2008;105:2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rizzoti K, Lovell-Badge R. SOX3 activity during pharyngeal segmentation is required for craniofacial morphogenesis. Development. 2007;134:3437–3448. [DOI] [PubMed] [Google Scholar]
- 41. Kelberman D, Rizzoti K, Avilion A, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116:2442–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woods KS, Cundall M, Turton J, et al. Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. Am J Hum Genet. 2005;76:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kiernan AE, Pelling AL, Leung KK, et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. [DOI] [PubMed] [Google Scholar]
- 44. Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36:247–255. [DOI] [PubMed] [Google Scholar]
- 45. Hoffmann SA, Hos D, Küspert M, et al. Stem cell factor Sox2 and its close relative Sox3 have differentiation functions in oligodendrocytes. Development. 2014;141:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sutton E, Hughes J, White S, et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest. 2011;121:328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6:1162–1168. [DOI] [PubMed] [Google Scholar]
- 48. Rogers CD, Harafuji N, Archer T, Cunningham DD, Casey ES. Xenopus Sox3 activates sox2 and geminin and indirectly represses Xvent2 expression to induce neural progenitor formation at the expense of non-neural ectodermal derivatives. Mech Dev. 2009;126:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr Opin Neurobiol. 2005;15:7–13. [DOI] [PubMed] [Google Scholar]
- 50. Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis. 1st ed Amsterdam, The Netherlands: North Holland Publishing; 1956. [Google Scholar]
- 51. Das B, Heimeier RA, Buchholz DR, Shi YB. Identification of direct thyroid hormone response genes reveals the earliest gene regulation programs during frog metamorphosis. J Biol Chem. 2009;284:34167–34178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hasebe T, Hartman R, Fu L, Amano T, Shi YB. Evidence for a cooperative role of gelatinase A and membrane type-1 matrix metalloproteinase during Xenopus laevis development. Mech Dev. 2007;124:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patterton D, Shi YB. Thyroid hormone-dependent differential regulation of multiple arginase genes during amphibian metamorphosis. J Biol Chem. 1994;269:25328–25334. [PubMed] [Google Scholar]
- 54. Sun G, Hasebe T, Fujimoto K, et al. Spatio-temporal expression profile of stem cell-associated gene LGR5 in the intestine during thyroid hormone-dependent metamorphosis in Xenopus laevis. PLoS One. 2010;5:e13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shi YB, Liang VC. Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim Biophys Acta. 1994;1217:227–228. [DOI] [PubMed] [Google Scholar]
- 56. Leloup J, Buscaglia M. La triiodothyronine: hormone de la métamorphose des amphibiens. C R Acad Sci. 1977;284:2261–2263. [Google Scholar]
- 57. Kanamori A, Brown DD. The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J Biol Chem. 1992;267:739–745. [PubMed] [Google Scholar]
- 58. McAvoy JW, Dixon KE. Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adult Xenopus laevis. J Exp Zool. 1977;202:129–138. [Google Scholar]
- 59. Ishizuya-Oka A, Ueda S. Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res. 1996;286:467–476. [DOI] [PubMed] [Google Scholar]
- 60. Ishizuya-Oka A, Ueda S, Damjanovski S, Li Q, Liang VC, Shi YB. Anteroposterior gradient of epithelial transformation during amphibian intestinal remodeling: immunohistochemical detection of intestinal fatty acid-binding protein. Dev Biol. 1997;192:149–161. [DOI] [PubMed] [Google Scholar]
- 61. López-Juárez A, Remaud S, Hassani Z, et al. Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell. 2012;10:531–543. [DOI] [PubMed] [Google Scholar]
- 62. Kress E, Samarut J, Plateroti M. Thyroid hormones and the control of cell proliferation or cell differentiation: paradox or duality? Mol Cell Endocrinol. 2009;313:36–49. [DOI] [PubMed] [Google Scholar]
- 63. Sirakov M, Plateroti M. The thyroid hormones and their nuclear receptors in the gut: from developmental biology to cancer. Biochim Biophys Acta. 2011;1812:938–946. [DOI] [PubMed] [Google Scholar]
- 64. Plateroti M, Kress E, Mori JI, Samarut J. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol Cell Biol. 2006;26:3204–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ishizuya-Oka A, Ueda S, Inokuchi T, et al. Thyroid hormone-induced expression of Sonic hedgehog correlates with adult epithelial development during remodeling of the Xenopus stomach and intestine. Differentiation. 2001;69:27–37. [DOI] [PubMed] [Google Scholar]
- 66. Hasebe T, Kajita M, Fu L, Shi YB, Ishizuya-Oka A. Thyroid hormone-induced sonic hedgehog signal up-regulates its own pathway in a paracrine manner in the Xenopus laevis intestine during metamorphosis. Dev Dyn. 2012;241:403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hasebe T, Kajita M, Shi YB, Ishizuya-Oka A. Thyroid hormone-up-regulated hedgehog interacting protein is involved in larval-to-adult intestinal remodeling by regulating sonic hedgehog signaling pathway in Xenopus laevis. Dev Dyn. 2008;237:3006–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stolow MA, Shi YB. Xenopus sonic hedgehog as a potential morphogen during embryogenesis and thyroid hormone-dependent metamorphosis. Nucleic Acids Res. 1995;23:2555–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pei D. Regulation of pluripotency and reprogramming by transcription factors. J Biol Chem. 2009;284:3365–3369. [DOI] [PubMed] [Google Scholar]
- 70. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 71. Fu L, Buchholz D, Shi YB. Novel double promoter approach for identification of transgenic animals: a tool for in vivo analysis of gene function and development of gene-based therapies. Mol Reprod Dev. 2002;62:470–476. [DOI] [PubMed] [Google Scholar]
- 72. Lei Y, Guo X, Deng Y, Chen Y, Zhao H. Generation of gene disruptions by transcription activator-like effector nucleases (TALENs) in Xenopus tropicalis embryos. Cell Biosci. 2013;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE. Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell. 1999;4:487–498. [DOI] [PubMed] [Google Scholar]
- 74. Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev. Genet. 2006;7:349–359. [DOI] [PubMed] [Google Scholar]