FIGURE 2.
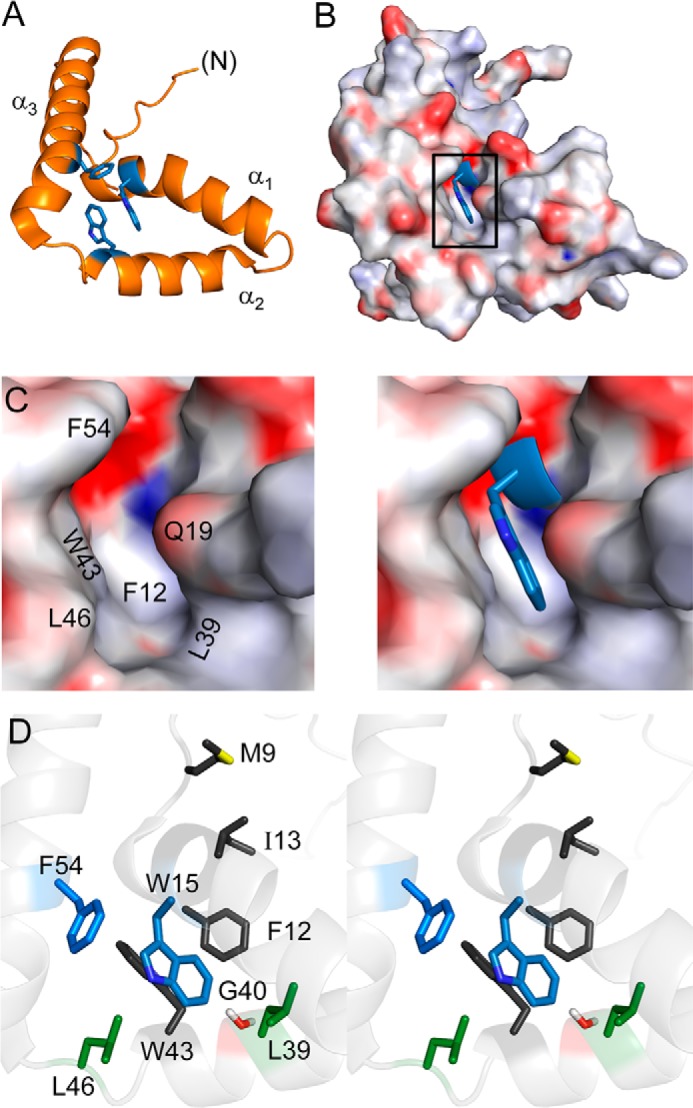
Structural environment of Trp-70 in human SRY. A, ribbon model of aromatic residues (blue) in major-wing core. B, docking of Trp-70 (box position 15; blue sticks) within a cavity (electrostatic surface in orientation shown in A). C and D, canonical structural relationships in the major wing of the SOX HMG box. Residue numbers refer to the HMG box consensus. C, expansion of boxed region in B. Left, electrostatic surface of the Trp-15-related cavity in absence of this side chain; Trp-15 main-chain atoms are shown in blue. Right, packing of Trp-15 side chain (blue sticks); the indole nitrogen is shown in a darker shade of blue. In this view, Phe-12 (residue 67 in human SRY) underlies Trp-15. D, side-chain packing in core (stereo). The side chains of Trp-15 and Phe-54 are shown in blue; “hydrophobic wedge” side chains Met-9, Phe-12, Ile-13, and Trp-43 in dark gray; Leu-39 and Leu-46 in green; and Gly-40 in red. Coordinates were obtained from PDB entry 1J46 (28).
