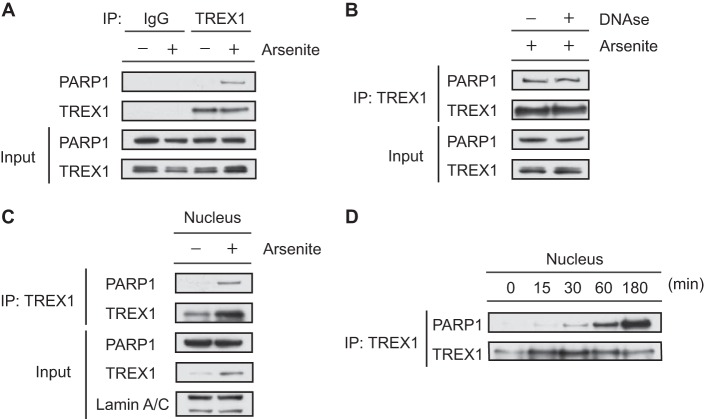
FIGURE 4.
Endogenous TREX1 interacts with PARP1 in response to DNA damage. A, whole-cell extracts from HeLa cells treated with or without sodium arsenite (2 mm) for 1 h were immunoprecipitated with anti-TREX1 or anti-rabbit IgG antibody and immunoblotted with anti-PARP1 or anti-TREX1 antibody. Input of the proteins was assessed by Western blotting with the indicated antibodies. B, DNase I was added to the extracts at 200 units for 1 h at 32 °C prior to co-IP of TREX1. C, nuclear extracts from HeLa cells were immunoprecipitated with anti-TREX1 antibody and immunoblotted with anti-PARP1 or anti-TREX1 antibody. Lamin A/C served as an endogenous loading control for nuclear proteins. D, nuclear extracts from HeLa cells treated with or without sodium arsenite (2 mm) for the indicated times were immunoprecipitated with anti-TREX1 antibody and immunoblotted with anti-PARP1 or anti-TREX1 antibody.