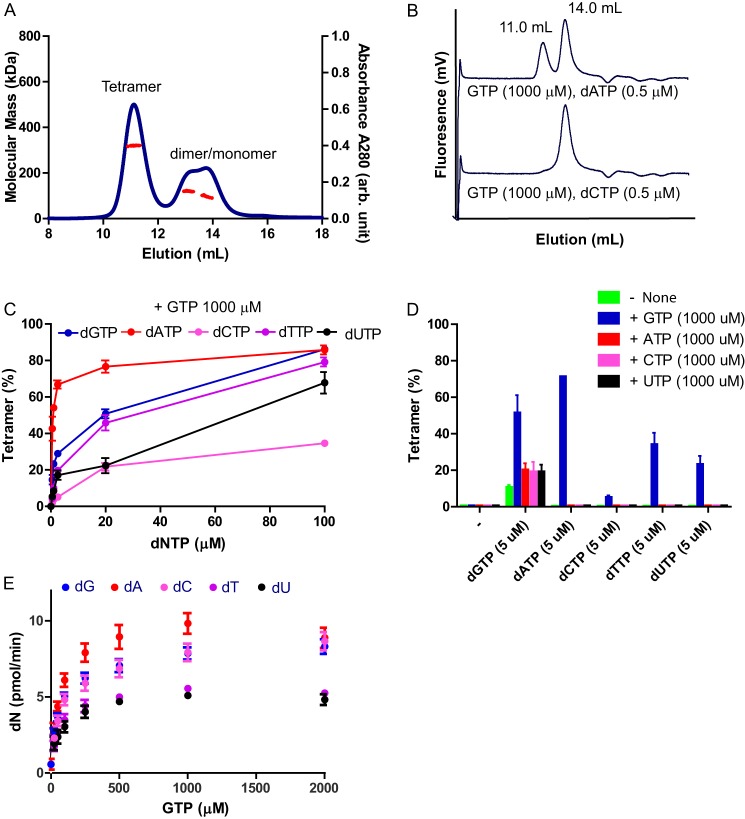
FIGURE 2.
SAMHD1 forms a tetramer with GTP and dNTPs and exhibits enzymatic activity. A, SEC-MALS of SAMHD1fl WT. The protein (25 μm) was preincubated with a mixture of GTP (250 μm) and dATP (250 μm). Molecular masses (red circles) of eluting species are shown across the A280 profile (blue circles). B, analytical gel filtration column chromatography of SAMHD1fl H210A (0.5 μm) with a mixture of GTP and dATP or dCTP. The elution volumes of SAMHD1 tetramer and dimer/monomer, based on SEC MALS experiments, are indicated. The catalytically inactive mutant was used for the assay to prevent hydrolysis of dNTP. C, SAMHD1fl H210A protein was preincubated with a mixture of GTP (at 1000 μm) and each dNTP as indicated (at 0, 0.5, 1.0, 2.5, 20, or 100 μm). The mixtures were separated by size exclusion column chromatography, and the data were analyzed as in B. D, SAMHD1fl H210A protein was preincubated with a mixture of one dNTP (at 5 μm) and/or one NTP (at 1000 μm), as indicated. The mixtures were analyzed as described in B. E, dNTPase activities of WT SAMHD1fl protein was measured using a mixture of dNTPs (each at 5 μm) and increasing concentrations of GTP (0, 25, 50, 100, 250, 500, 1000, and 2000 μm). Each experiment was performed in triplicate. Error bars, S.D. for the individual experiments.