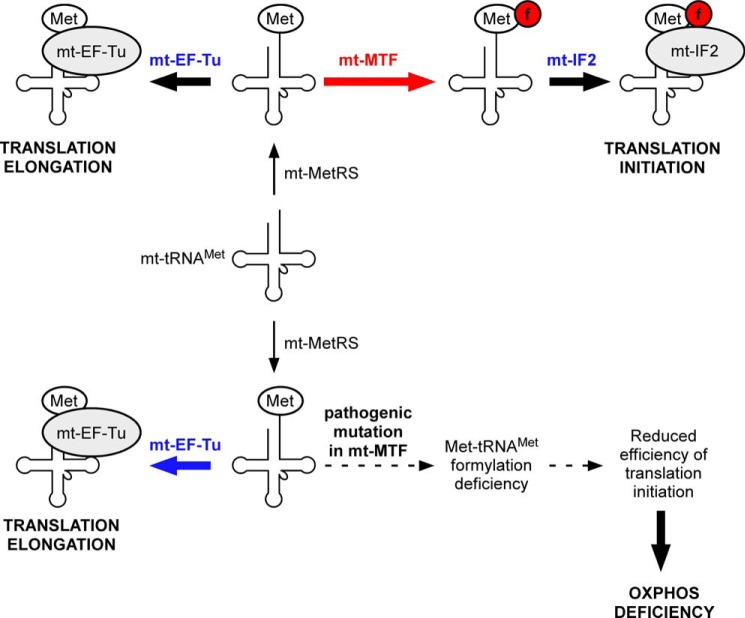
FIGURE 1.
Top, schematic representation depicting the use of a single mt-tRNAMet for both translation initiation and elongation. The mt-tRNAMet is aminoacylated by mt-MetRS to form Met-tRNAMet. A fraction of Met-tRNAMet is formylated by mt-MTF to generate fMet-tRNAMet, which interacts with mt-IF2 to participate in translation initiation. The remaining fraction of Met-tRNAMet binds to mt-EF-Tu, which transports it to the mt-ribosome for use in translation elongation. Bottom, mutations in mt-MTF causing reduction in its activity can have a deleterious effect on translation initiation because mt-MTF and mt-EF-Tu compete for the same mitochondrial Met-tRNAMet. Because of this competition, even small changes in the activity or levels of mt-MTF could potentially cause a significant reduction in mitochondrial translation initiation, leading to different forms of oxidative phosphorylation deficiency diseases.