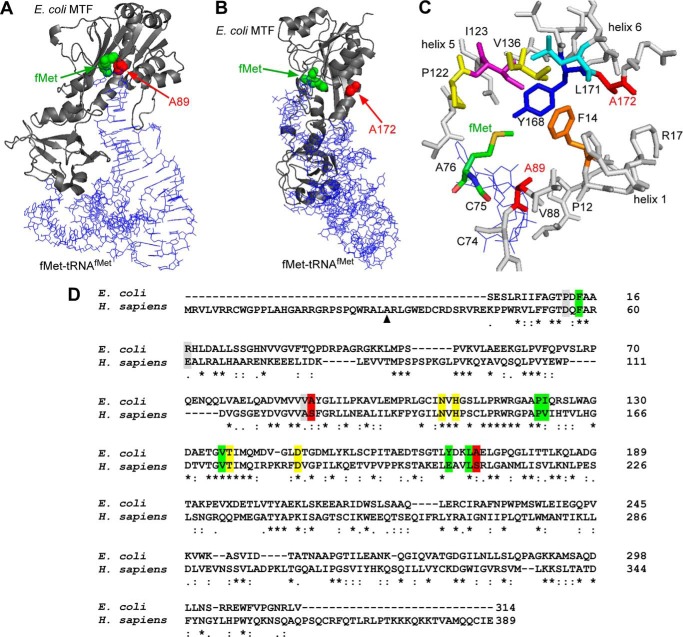
FIGURE 7.
Structural representation of Ala-89 and Ala-172 in the context of the E. coli MTF-fMet-tRNAfMet complex. Structures were drawn using PyMOL (Schrödinger, LLC, New York) and Protein Data Bank entry 2FMT. A, a backbone trace of the complex is depicted, where E. coli MTF is shown in a ribbon representation (gray) and the tRNA molecule is shown as lines (blue). The positions of the fMet moiety and Ala-89 in the methionine binding pocket are highlighted with green and red spheres, respectively. B, the same complex is shown from a different angle to highlight the positions of fMet and Ala-172, as indicated by green and red spheres, respectively. The Ala-172 residue is distal to the methionine binding pocket and belongs to helix 6. C, close-up view of amino acids (represented as sticks) lining the methionine binding pocket of E. coli MTF. The CCA end (Cys-74, Cys-75, and Cys-76) of the initiator tRNA is shown as blue lines. D, pairwise alignment of E. coli MTF and mt-MTF using ClustalW2. Ala-89 and Ala-172 (Ser-125 and Ser-209 in mt-MTF) are highlighted in red. Green, amino acids lining the methionine binding pocket (Phe-14, Ile-123, Val-136, Leu-171, Pro-122, and Tyr-168). Note that Ala-89 is part of the methionine binding pocket (39). Yellow, amino acids involved in FTHF binding and catalysis (Asn-108, His-110, Thr-137, and Asp-146) (38). Gray, additional amino acids of interest mentioned in this work (Pro-12, Val-88, and Arg-17). The numbering of residues in E. coli MTF starts with the serine residue at the N terminus of the mature protein. The numbering for human mt-MTF starts with the first amino acid methionine as encoded by the mt-MTF gene. ▴, start site of mt-MTF after removal of the mitochondrial targeting site.