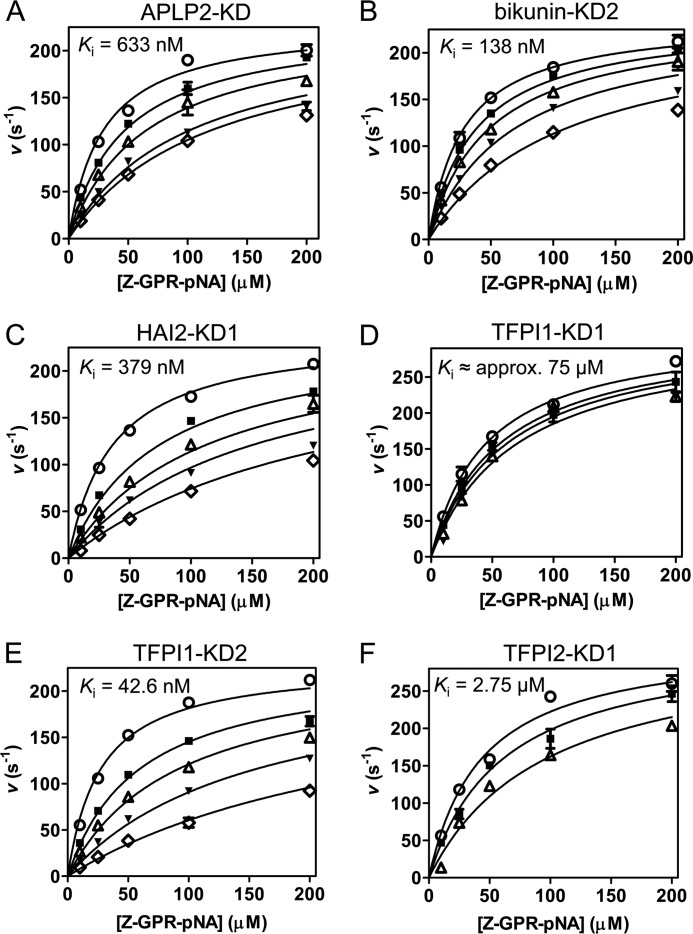
FIGURE 2.
Competitive inhibition of mesotrypsin by recombinant Kunitz domains. For each Kunitz domain, initial reaction rates are plotted for a representative experiment in which peptide substrate Z-GPR-pNA concentration and Kunitz domain concentration were varied; fitted lines obtained from multiple regression using the competitive inhibition equation are superposed on the data. All studies were performed with a final mesotrypsin concentration of 0.25 nm and with substrate concentration ranging from 10 to 200 μm as indicated on the x axis. A, mesotrypsin is competitively inhibited by APLP2-KD with a Ki of 633 nm. APLP2 concentration was 0 nm (○), 400 nm (■), 800 nm (▵), 1600 nm (▾), or 2000 nm (♢). B, mesotrypsin is competitively inhibited by bikunin-KD2 with a Ki of 138 nm. Bikunin-KD2 concentration was 0 nm (○), 50 nm (■), 100 nm (▵), 200 nm (▾), or 400 nm (♢). C, HAI2-KD1 competitively inhibits mesotrypsin with a Ki of 379 nm. HAI2-KD1 concentration was 0 nm (○), 400 nm (■), 800 nm (▵), 1200 nm (▾), or 2000 nm (♢). D, mesotrypsin is competitively inhibited by TFPI1-KD1 with a Ki of ∼75 μm. Concentrations of TFPI1-KD1 were 0 μm (○), 20 μm (■), 30 μm (▵), or 45 μm (▾). Higher concentrations of TFPI1-KD1 could not be tested due to the limited quantities of recombinant protein available, and thus the calculated Ki represents a rough approximation. E, TFPI1-KD2 competitively inhibits mesotrypsin with a Ki of 42.6 nm. TFPI1-KD2 concentrations were 0 nm (○), 50 nm (■), 100 nm (▵), 200 nm (▾), or 400 nm (♢). F, TFPI2-KD1 competitively inhibits mesotrypsin with a Ki of 2.75 μm. TFPI2-KD1 concentrations were 0 μm (○), 1 μm (■), or 3 μm (▵). Error bars, S.E.