FIGURE 6.
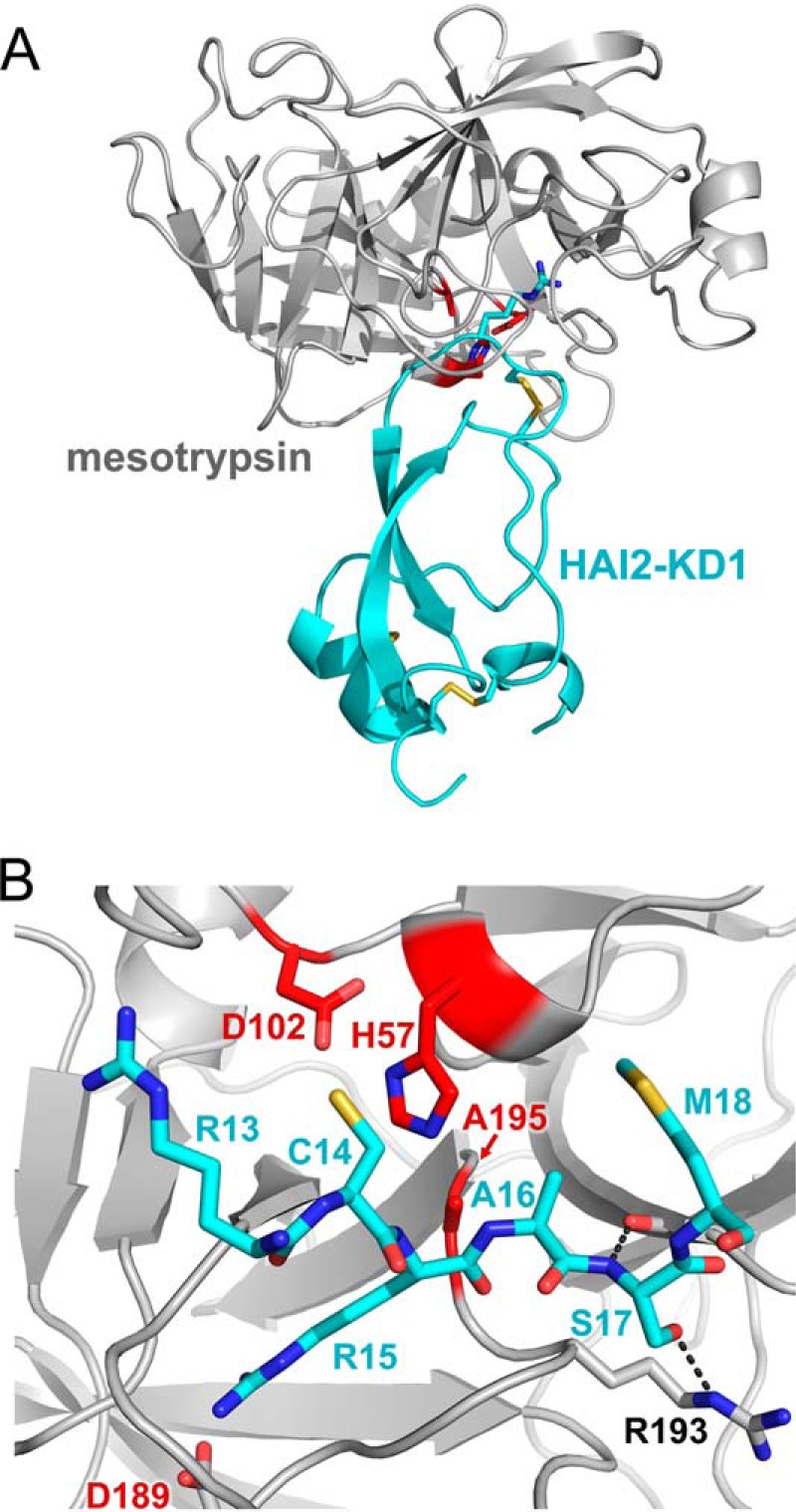
Structure of the mesotrypsin·HAI2-KD1 complex. A, structural overview shows mesotrypsin in a light gray schematic representation bound to HAI2-KD1 in cyan. Catalytic triad residues of the mesotrypsin active site are colored red, and Arg15 of the HAI2-KD1 reactive site, positioned in the S1 specificity pocket of mesotrypsin, is rendered in a stick representation. B, mesotrypsin active site occupied by HAI2-KD1 P3–P′3 residues is shown in standard orientation, with nonprimed residues on the left and primed residues on the right. The HAI2-KD1 Arg15-Ala16 reactive site bond is positioned as would be expected for cleavage by the mesotrypsin catalytic triad of Ser195 (in our structure mutated to Ala), His57, and Asp102. Black dashed lines illustrate hydrogen bonds formed between HAI2-KD1 Ser17 nitrogen and mesotrypsin Phe41 oxygen and between HAI2-KD1 Ser17 Oγ and mesotrypsin Arg193 Nϵ.
