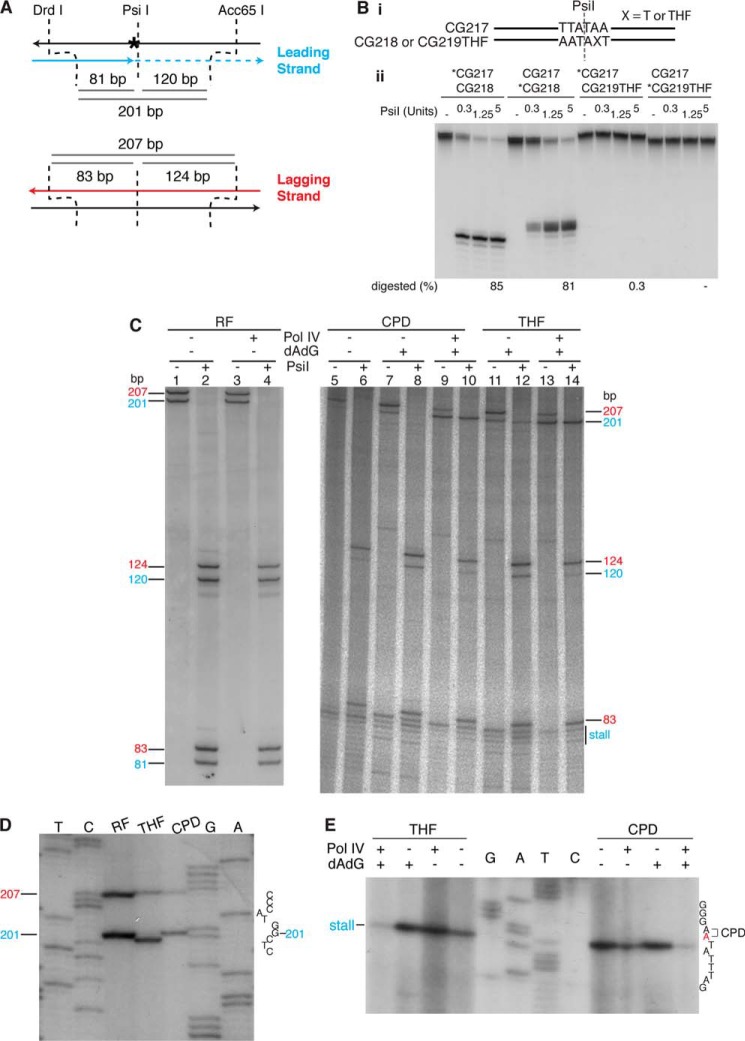
FIGURE 6.
Pol IV-catalyzed, replisome-mediated TLS bypass generates a −1 frameshift on the THF template. A, scheme for analyzing the nascent DNA products. B, a THF lesion inhibits digestion of DNA by the PsiI restriction endonuclease. Panel i, schematic of the two duplex DNA oligonucleotides used as substrates. The DNA sequence is identical to that about the site of the lesion in the template DNA used for replication. Panel ii, different combinations of oligonucleotides were treated with PsiI as indicated, and the DNA products were analyzed by denaturing polyacrylamide gel electrophoresis as described under “Experimental Procedures.” An asterisk on an oligonucleotide name denotes that it was 5′-[32P] end-labeled. C, leading strand replication is contiguous across the site of the template lesion. DNA products generated by replication with either the undamaged, CPD, or THF template either in the presence or absence of Pol IV (100 nm) and the presence or absence of elevated concentrations (0.75 mm) of dATP and dGTP were digested with DrdI and Acc65I either with or without digestion with PsiI as indicated, processed, and analyzed by electrophoresis through a denaturing polyacrylamide gel as described under “Experimental Procedures.” RF, replicative form DNA. D, Pol IV-catalyzed, replisome-mediated TLS bypass generates a −1 frameshift on the THF template. DNA products generated as described for C in the presence of Pol IV (100 nm), and elevated nucleotide concentrations (0.75 mm) were digested with DrdI and Acc65I and analyzed by electrophoresis through a high resolution denaturing 6% polyacrylamide gel (19:1, acrylamide:bisacrylamide) as described under “Experimental Procedures.” T, C, G, and A show a DNA sequencing ladder prepared from undamaged DNA using a primer that has the same 5′ end as the DrdI-digested nascent leading strand DNA. For reasons that are unclear, migration of the digested nascent leading strand from the CPD template was somewhat variable compared with the product from the undamaged template; it could have the same mobility or be a bit slower. We think this could be because of differences in loading volumes necessitated by the differences in the extent of replication with the two templates. E, leading strand replication stalls just 5′ of the template lesion. DNA products generated using either the THF or CPD templates, either in the presence or absence of Pol IV (100 nm) and either in the presence or absence of elevated concentrations (0.75 mm) of dATP and dGTP were prepared and analyzed as in D except they were digested only with DrdI.