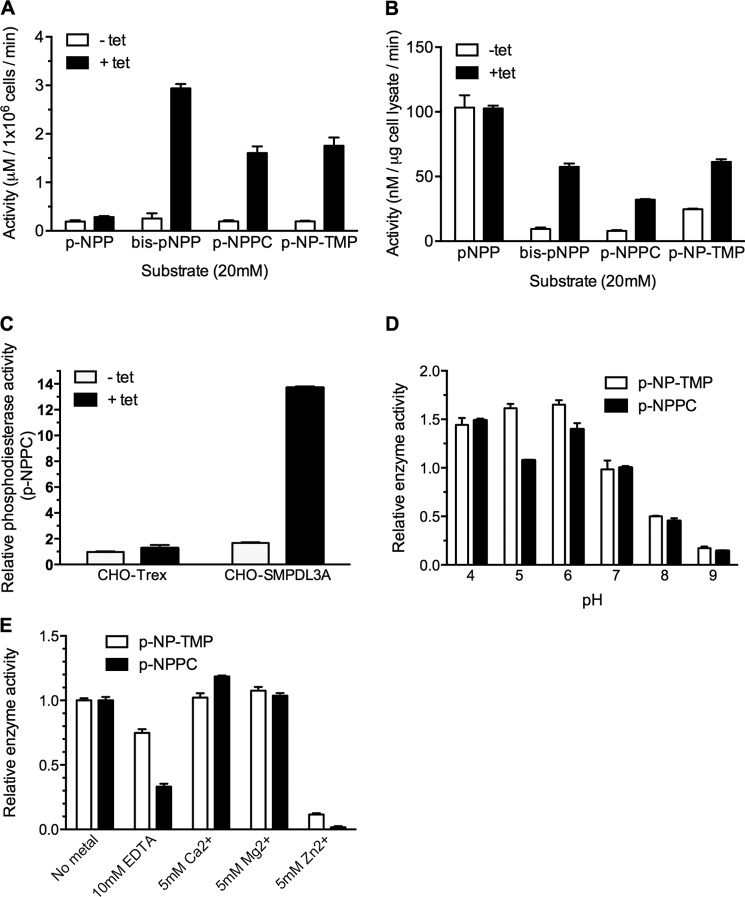
FIGURE 6.
Activity of recombinant human SMPDL3A against synthetic p-nitrophenyl phosphodiester substrates. A, activity of conditioned serum-free media harvested from 1 × 106 control or tetracycline-induced CHO-SMPDL3A cells against p-NPP, bis-p-NPP, p-NPPC, and p-NP-TMP. In each case, the final substrate concentration was 20 mm, in 125 mm Tris, pH 7.0, 5 mm Mg2+. B, activity of cell lysates from control or tetracycline-induced CHO-SMPDL3A cells against substrates listed in A. C, parental nontransfected CHO-Trex or transfected CHO-SMPDL3A cells were treated with or without 1 μm tetracycline, and relative phosphodiesterase activity against 20 mm p-NPPC substrate was measured in whole cell lysates (125 mm Tris, pH 7.0, 5 mm Mg2+, 1 h at 37 °C). Activity values were normalized to noninduced CHO-Trex cell lysates. Effect of pH (D) or EDTA/metal ions (E) on relative activity against 20 mm p-NP-TMP and p-NPPC for purified recombinant SMPDL3A is shown. Activity values were normalized to p-NP-TMP at pH 7 or no metal supplementation, respectively. For all experiments involving cell lysates or conditioned media, tetracycline induction was 1 μm final concentration for 16 h. Data are means ± S.D. of three determinations.