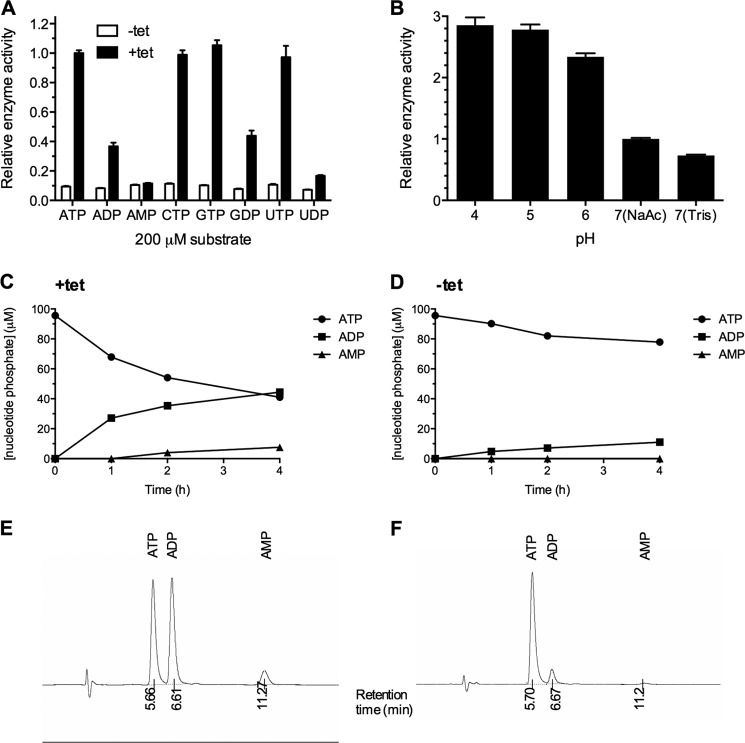
FIGURE 8.
A, nucleotide phosphodiesterase activity of recombinant SMPDL3A against nucleotide phosphate substrates. The activity of concentrated and buffer-exchanged conditioned media from CHO-SMPDL3A cells treated with or without 1 μm tetracycline for 16 h was determined against a panel of nucleotide phosphates. Activity was measured by colorimetric detection at 620 nm of released inorganic phosphate. Concentrated conditioned medium was mixed with reaction buffer in a 1:1 v/v ratio. Final reaction conditions were 100 mm Tris, pH 7.0, 5 mm Mg2+, and 200 μm nucleotide phosphate. Data are means ± S.D. of three determinations. B, pH dependence of purified recombinant SMPDL3A on ATP hydrolytic activity (100 mm sodium acetate, pH 4–7, 100 mm Tris, pH 7). Reverse phase HPLC analysis of reaction products after incubation of ATP with conditioned media from tetracycline-induced (C) and noninduced control (D) CHO-SMPDL3A cells. Reaction mixture was 100 μm ATP, 100 mm sodium acetate, pH 7. Detection of nucleotide products was performed by measuring absorbance at 254 nm. Representative chromatograms showing retention times for nucleotide species for induced and noninduced samples are shown in E and F, respectively.