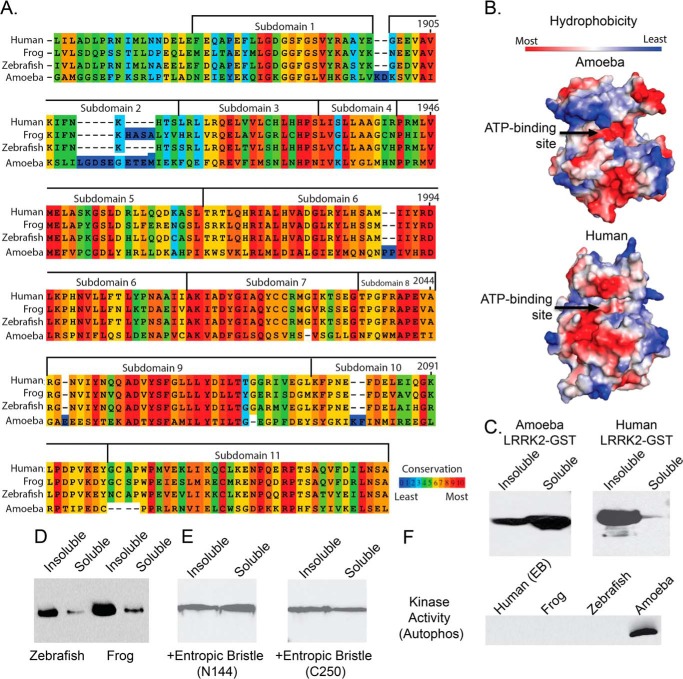
FIGURE 8.
Poor conservation and insolubility of the mammalian LRRK2 ATP-binding pocket. A, kinase domain similarity between the human and frog (88% ATP pocket identity), zebrafish (72% kinase domain similarity), and ameba (31% ATP pocket identity) LRRK2 kinase domains. B, hydrophobicity space fill of the ameba LRRK2 kinase domain crystal structure compared with the imposed LRRK2 human kinase domain sequence on this structure. Arrows indicate the ATP-binding pocket. C and D, representative comparison of solubility profiles of the isolated kinase domains expressed by E. coli. for ameba and human (C) and for the zebrafish and frog LRRK2 kinase domains (D). E and F, recovery of the human LRRK2 kinase domain solubility with the inclusion of either an N-terminal (Asn-144) or C-terminal (Cys-250) entropic bristle (EB) tag (E) but failure of this protein or higher-order LRRK2 kinase domain proteins (frog and zebrafish) (F) to demonstrate any kinase activity.