Abstract
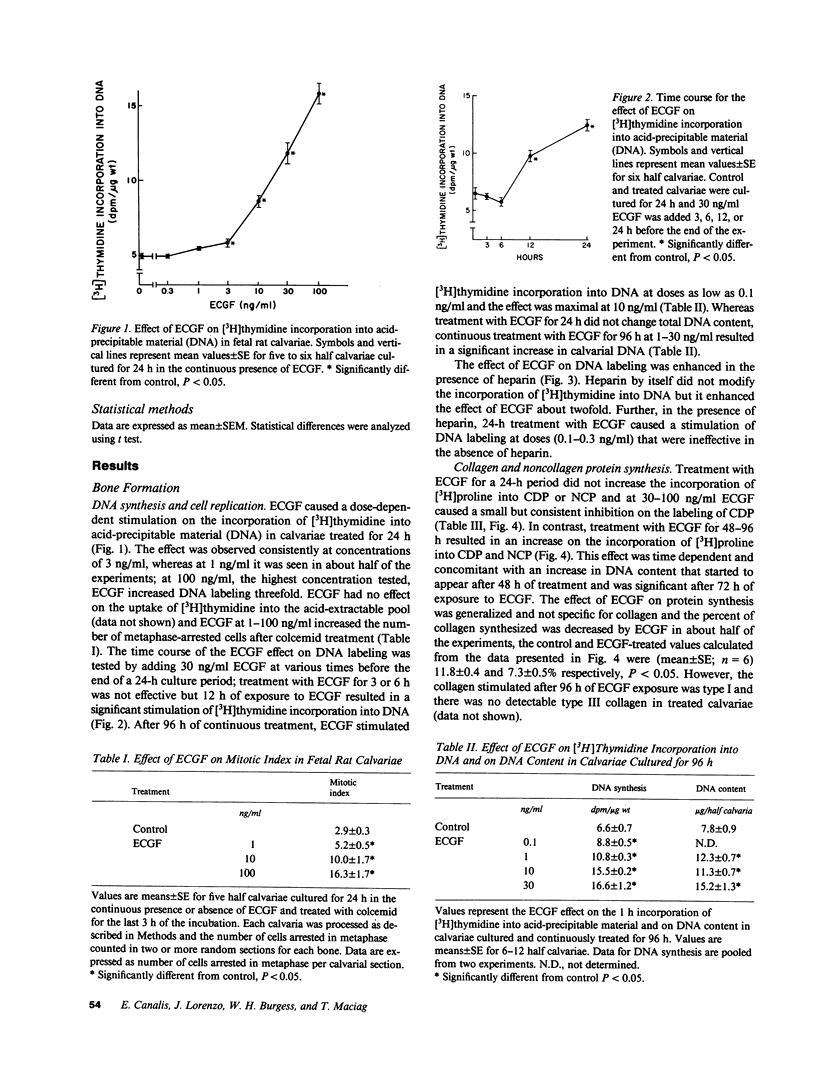
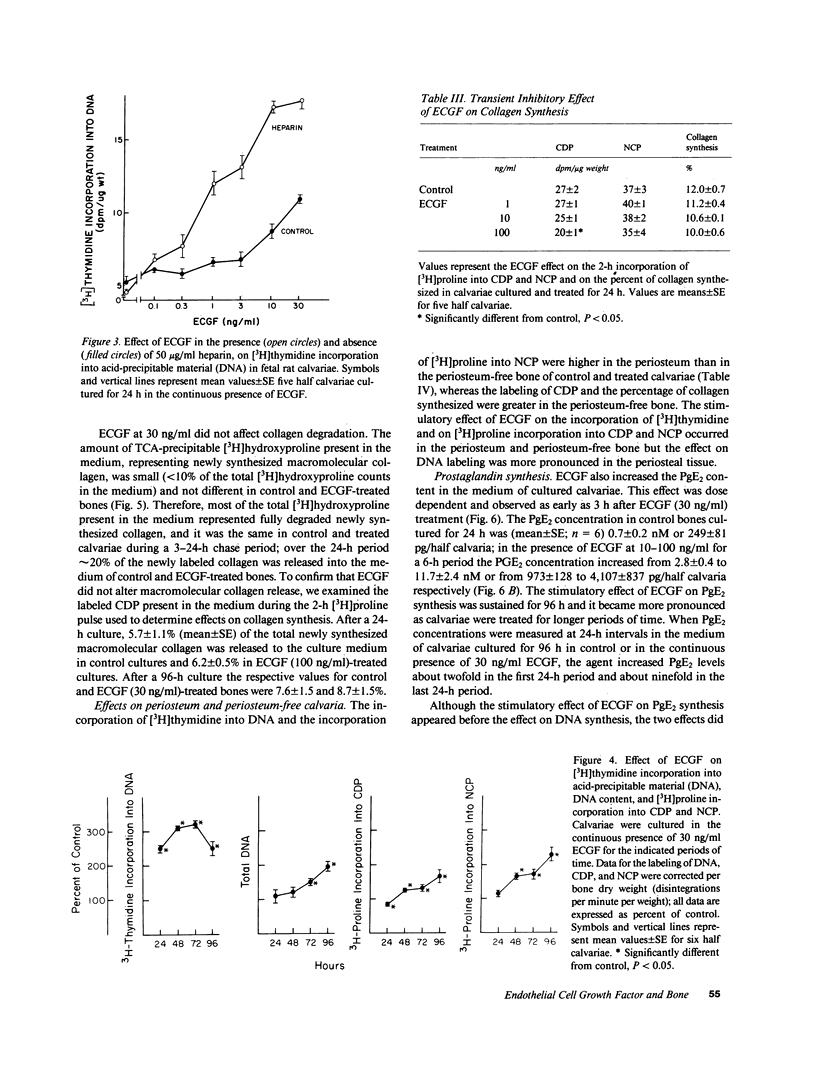
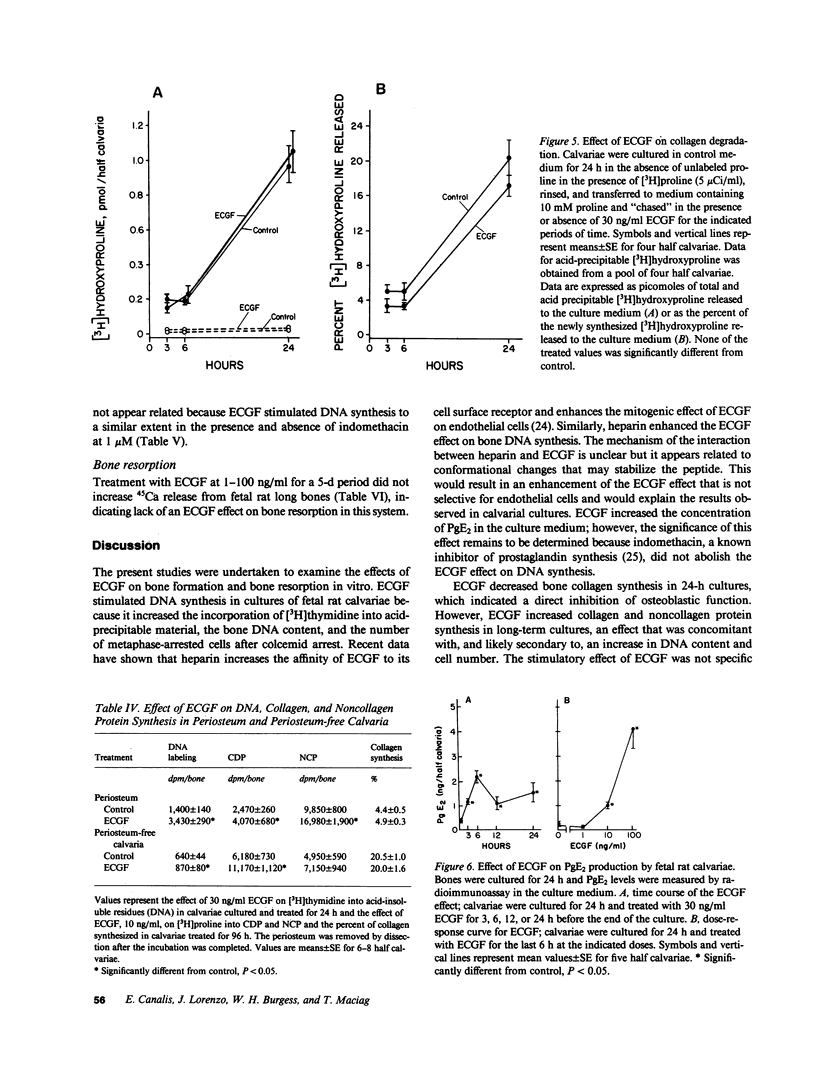
Endothelial cell growth factor (ECGF) alpha was studied for its effects on bone formation in cultured fetal rat calvariae and on bone resorption in cultured fetal rat long bones. ECGF at 0.1-100 ng/ml stimulated [3H]thymidine incorporation into DNA, an effect enhanced by heparin. Treatment with ECGF for 24 h decreased the incorporation of [3H]proline into collagen but treatment for 48-96 h increased collagen and noncollagen protein synthesis, an effect that was concomitant with an increase in DNA content. ECGF did not alter collagen degradation in calvariae or 45Ca release from long bones, which indicated it had no effect on bone resorption. Although ECGF increased prostaglandin E2 concentrations, its effect on DNA synthesis was not prostaglandin-mediated. In conclusion, ECGF stimulates calvarial DNA synthesis, which is an effect that results in a generalized increase in protein synthesis, but ECGF has no effect on matrix degradation or bone resorption.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess W. H., Mehlman T., Friesel R., Johnson W. V., Maciag T. Multiple forms of endothelial cell growth factor. Rapid isolation and biological and chemical characterization. J Biol Chem. 1985 Sep 25;260(21):11389–11392. [PubMed] [Google Scholar]
- Canalis E., Centrella M. Isolation of a nontransforming bone-derived growth factor from medium conditioned by fetal rat calvariae. Endocrinology. 1986 May;118(5):2002–2008. doi: 10.1210/endo-118-5-2002. [DOI] [PubMed] [Google Scholar]
- Canalis E. Effect of glucocorticoids on type I collagen synthesis, alkaline phosphatase activity, and deoxyribonucleic acid content in cultured rat calvariae. Endocrinology. 1983 Mar;112(3):931–939. doi: 10.1210/endo-112-3-931. [DOI] [PubMed] [Google Scholar]
- Canalis E. Effect of growth factors on bone cell replication and differentiation. Clin Orthop Relat Res. 1985 Mar;(193):246–263. [PubMed] [Google Scholar]
- Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest. 1980 Oct;66(4):709–719. doi: 10.1172/JCI109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E. Effect of platelet-derived growth factor on DNA and protein synthesis in cultured rat calvaria. Metabolism. 1981 Oct;30(10):970–975. doi: 10.1016/0026-0495(81)90094-9. [DOI] [PubMed] [Google Scholar]
- Canalis E. Interleukin-1 has independent effects on deoxyribonucleic acid and collagen synthesis in cultures of rat calvariae. Endocrinology. 1986 Jan;118(1):74–81. doi: 10.1210/endo-118-1-74. [DOI] [PubMed] [Google Scholar]
- Canalis E., Peck W. A., Raisz L. G. Stimulation of DNA and collagen synthesis by autologous growth factor in cultured fetal rat calvaria. Science. 1980 Nov 28;210(4473):1021–1023. doi: 10.1126/science.7434011. [DOI] [PubMed] [Google Scholar]
- Canalis E., Raisz L. G. Effect of epidermal growth factor on bone formation in vitro. Endocrinology. 1979 Apr;104(4):862–869. doi: 10.1210/endo-104-4-862. [DOI] [PubMed] [Google Scholar]
- Canalis E. The hormonal and local regulation of bone formation. Endocr Rev. 1983 Winter;4(1):62–77. doi: 10.1210/edrv-4-1-62. [DOI] [PubMed] [Google Scholar]
- Centrella M., Canalis E. Local regulators of skeletal growth: a perspective. Endocr Rev. 1985 Fall;6(4):544–551. doi: 10.1210/edrv-6-4-544. [DOI] [PubMed] [Google Scholar]
- Centrella M., Massagué J., Canalis E. Human platelet-derived transforming growth factor-beta stimulates parameters of bone growth in fetal rat calvariae. Endocrinology. 1986 Nov;119(5):2306–2312. doi: 10.1210/endo-119-5-2306. [DOI] [PubMed] [Google Scholar]
- Esch F., Ueno N., Baird A., Hill F., Denoroy L., Ling N., Gospodarowicz D., Guillemin R. Primary structure of bovine brain acidic fibroblast growth factor (FGF). Biochem Biophys Res Commun. 1985 Dec 17;133(2):554–562. doi: 10.1016/0006-291x(85)90942-8. [DOI] [PubMed] [Google Scholar]
- Gimenez-Gallego G., Rodkey J., Bennett C., Rios-Candelore M., DiSalvo J., Thomas K. Brain-derived acidic fibroblast growth factor: complete amino acid sequence and homologies. Science. 1985 Dec 20;230(4732):1385–1388. doi: 10.1126/science.4071057. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Levine L., Hinkle P. M., Voelkel E. F., Tashjian A. H., Jr Prostaglandin production by mouse fibrosarcoma cells in culture: inhibition by indomethacin and aspirin. Biochem Biophys Res Commun. 1972 May 26;47(4):888–896. doi: 10.1016/0006-291x(72)90576-1. [DOI] [PubMed] [Google Scholar]
- Lindholm R., Lindholm S., Liukko P., Paasimäki J., Isokäntä S., Rossi R., Autio E., Tamminen E. The mast cell as a component of callus in healing fractures. J Bone Joint Surg Br. 1969 Feb 1;51(1):148–155. [PubMed] [Google Scholar]
- Lobb R., Sasse J., Sullivan R., Shing Y., D'Amore P., Jacobs J., Klagsbrun M. Purification and characterization of heparin-binding endothelial cell growth factors. J Biol Chem. 1986 Feb 5;261(4):1924–1928. [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Isgaard J., Lindahl A., Dahlström A., Skottner A., Isaksson O. G. Regulation by growth hormone of number of chondrocytes containing IGF-I in rat growth plate. Science. 1986 Aug 1;233(4763):571–574. doi: 10.1126/science.3523759. [DOI] [PubMed] [Google Scholar]
- PECK W. A., BIRGE S. J., Jr, FEDAK S. A. BONE CELLS: BIOCHEMICAL AND BIOLOGICAL STUDIES AFTER ENZYMATIC ISOLATION. Science. 1964 Dec 11;146(3650):1476–1477. doi: 10.1126/science.146.3650.1476. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Niemann I. Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology. 1969 Sep;85(3):446–452. doi: 10.1210/endo-85-3-446. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Simmons H. A., Sandberg A. L., Canalis E. Direct stimulation of bone resorption by epidermal growth factor. Endocrinology. 1980 Jul;107(1):270–273. doi: 10.1210/endo-107-1-270. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Kenney J., Kowalski J., Thomas K. A., Gimenez-Gallego G., Rios-Candelore M., Di Salvo J., Barritault D., Courty J., Courtois Y. A unique family of endothelial cell polypeptide mitogens: the antigenic and receptor cross-reactivity of bovine endothelial cell growth factor, brain-derived acidic fibroblast growth factor, and eye-derived growth factor-II. J Cell Biol. 1985 Oct;101(4):1623–1626. doi: 10.1083/jcb.101.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Kenney J., Kowalski W. J., Friesel R., Mehlman T., Maciag T. Interaction of endothelial cell growth factor with heparin: characterization by receptor and antibody recognition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6138–6142. doi: 10.1073/pnas.82.18.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedin S. M., Thompson A. Y., Bentz H., Rosen D. M., McPherson J. M., Conti A., Siegel N. R., Galluppi G. R., Piez K. A. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J Biol Chem. 1986 May 5;261(13):5693–5695. [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lazzaro M., Singer F. R., Roberts A. B., Derynck R., Winkler M. E., Levine L. Alpha and beta human transforming growth factors stimulate prostaglandin production and bone resorption in cultured mouse calvaria. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4535–4538. doi: 10.1073/pnas.82.13.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Giménez-Gallego G., DiSalvo J., Bennett C., Rodkey J., Fitzpatrick S. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6409–6413. doi: 10.1073/pnas.82.19.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist M. R., DeLange R. J., Finerman G. A. Bone cell differentiation and growth factors. Science. 1983 May 13;220(4598):680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- Wong G. L., Cohn D. V. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3167–3171. doi: 10.1073/pnas.72.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]


