Figure 2.
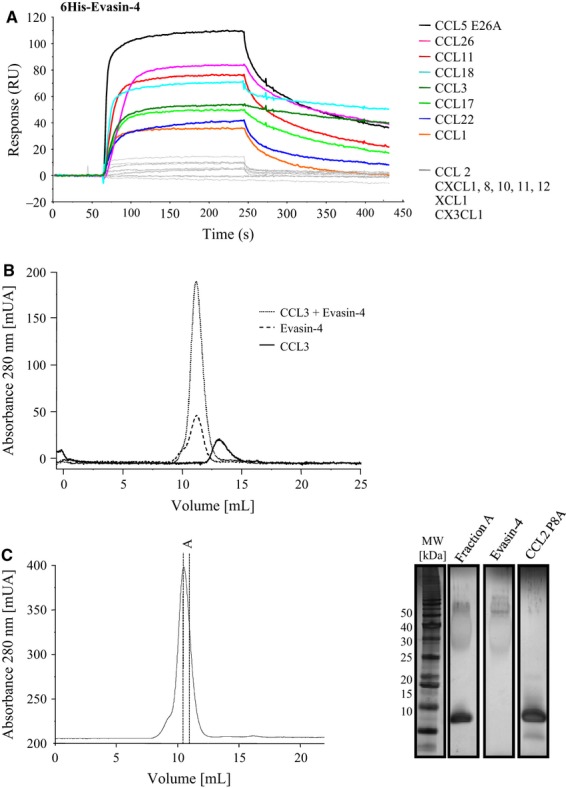
Selectivity of recombinant Evasin-4. (A) Chemokines suspended at 0.1 μg·mL−1 in running buffer were injected for 3 min on coated 6His–Evasin-4 followed by 2.5 min of dissociation. CCL5 was replaced by the mutant CCL5 E26A to avoid oligomerization 27. CCL1, -3, -5, -11, -17, -18, -22 and -26 showed strong binding to Evasin-4, whereas sensograms of CCL2, CXCL1, -8, -10, -11, -12, XCL1 and CX3CL1 indicated no binding of these chemokines to Evasin-4. (B) Complex formation between Evasin-4 and human CCL3. Evasin-4, CCL3 and an equimolar mixture of the two proteins were subjected one by one to SEC. Observation of a single peak when analyzing the mixture confirmed the formation of a complex between the two proteins. (C) SEC analysis of the complex between Evasin-4 and the obligate monomer CCL2 P8A. Equimolar amounts of Evasin-4 and CCL2 P8A were incubated and loaded onto a SEC column (left). A fraction of the peak analyzed by SDS/PAGE followed by silver staining confirmed the presence of both Evasin-4 and CCL2 (right).
