Figure 4.
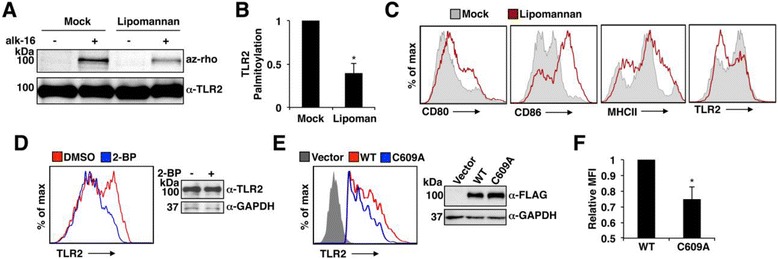
S-palmitoylation promotes TLR2 cell surface expression. A-C) BMDCs were mock treated or treated with 4 ug/mL lipomannan for 24 hours. A) Cells were labeled with 50 uM alk-16 for one hour. Immunoprecipitated TLR2 was reacted with az-rho for visualization of palmitoylation. Anti-TLR2 Western blotting confirmed comparable protein loading. B) Fluorescent gel scans from four experiments as in A were quantified and normalized to their respective Western blots. Values for lipomannan were normalized relative to a value set to 1 for mock, and were averaged. The error bar represents the standard deviation of four experiments. *P <0.001 by Student’s t-test. C) Cells were stained with antibodies against the indicated surface proteins and analyzed by flow cytometry. Results in C are representative of at least three experiments. D) BMDCs were treated with 100 uM 2-BP or solvent control for 12 hours, and stained with anti-TLR2 antibody for flow cytometry analysis of TLR2 surface levels, or were lysed and subjected to anti-TLR2 Western blotting to examine total TLR2 levels. Anti-GAPDH blotting served as a loading control. Results in D are representative of at least three similar experiments. E, F) MEFs were transfected with plasmids expressing murine FLAG-TLR2 (WT), FLAG-TLR2-C609A (C609A), or vector control. E) Cells were subjected to anti-TLR2 staining for flow cytometry analysis of TLR2 surface expression or were lysed for western blotting with anti-FLAG antibodies for comparing total TLR2 protein levels. Anti-GAPDH Western blotting was performed as a loading control. F) Flow cytometry results from three experiments as in E were quantified in terms of mean fluorescence intensity (MFI). Values for C609A were normalized relative to a value set to 1 for WT, and were averaged. The error bar represents the standard deviation of three experiments. *P <0.01 by Student’s t-test. az-rho, azido-rhodamne; BMDCs, bone marrow dendritic cells; MEFs, murine embryonic fibroblasts; TLR, Toll-like receptor.
