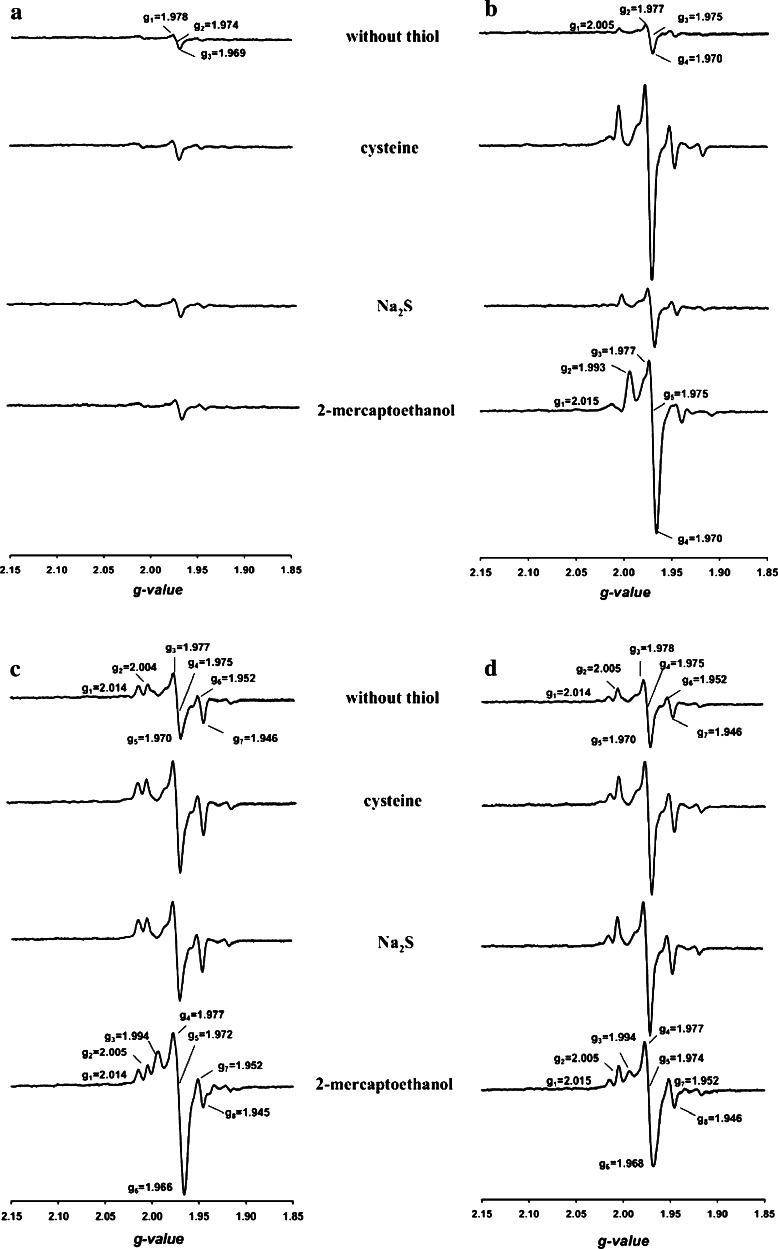
Fig. 8.
Mo-EPR of CO dehydrogenases in the presence of l-cysteine (15 mM), sodium sulfide (15 µM) or 2-mercaptoethanol (15 mM). a Enzyme from wild-type bacteria was treated with 5 mM potassium cyanide, small molecules were removed by gel filtration, and sulfur compounds were added as indicated. b Wild-type enzyme treated with 5 mM potassium cyanide was sulfurated (5 mM sodium sulfide plus 5 mM sodium dithionite) and, after gel filtration, supplied with the indicated sulfur compounds. The enzymes from the mutant E::km (c) or F::km (d) in their as isolated state were supplied with the indicated sulfur compounds. All assays contained CO dehydrogenase (12 mg ml−1) in 50 mM HEPES (pH 7.2) sparged with pure N2. EPR spectra were recorded as detailed in the legend to Fig. 7