Figure 2.
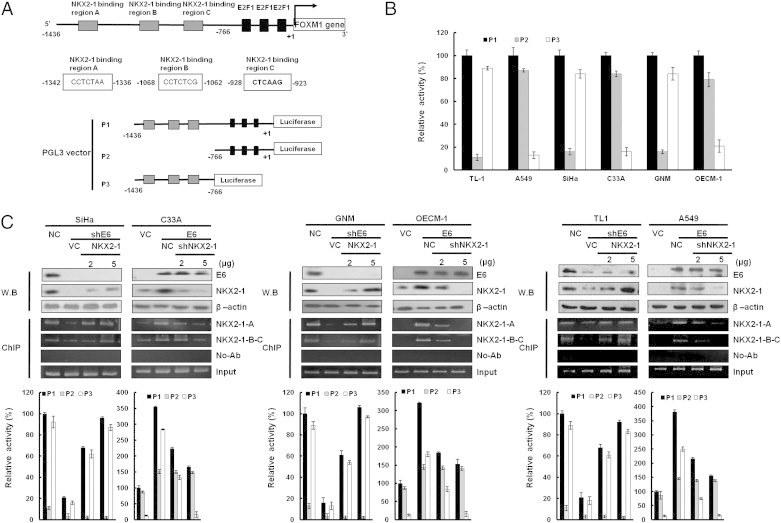
Up-regulation of FOXM1 transcription by E6 is through increased NKX2-1 binding to the FOXM1 promoter. (A) Schematic diagram of FOXM1 promoter–driven luciferase reporters: FOXM1 (− 1436/+ 1)-Luc, FOXM1 (− 766/+ 1)-Luc, and FOXM1 (− 1436/-766)-Luc. NKX2-1-A, NKX2-1-B, and NKX2-1-C possess putative NKX2-1 binding sites. (B) The three promoter constructs were co-transfected into SiHa, C33A, GNM, OECM-1, TL-1, and A549 cells for luciferase reporter assay after 48 hours. Luciferase activity was measured at 48-hour post-transfection. In all experiments, the relative luciferase activity was shown as the fold activation relative to that of the control cells. (C) SiHa, GNM, and TL-1 cells were transfected with various doses of E6-knockdown plasmid as indicated. C33A, OECM-1, and A549 cells were transfected with various doses of E6-overexpressing plasmid. The total amount of transfected DNA was kept constant by adding a control vector. The luciferase activity was measured at 48-hour post-transfection. In all experiments, the relative luciferase activity was shown as the fold activation relative to that of the control cells. SiHa, GNM, and TL-1 cells were transfected with various doses of E6-knockdown plasmid as indicated. C33A, OECM-1, and A549 cells were transfected with various doses of E6-overexpressing plasmid. In addition, SiHa, GNM, and TL-1 cells were co-transfected with NKX2-1-overexpressing (5 μg) and E6-knockdown (5 μg) plasmids. C33A, OECM-1, and A549 cells were co-transfected with shNKX2-1-knockdown (5 μg) and E6-overexpressing (5 μg) plasmids. These lysates were immunoprecipitated with anti–NKX2-1-conjugated beads. The immunoprecipitates were analyzed through SDS-PAGE, followed by immunoblot analysis with anti–NKX2-1 antibody. The input control was 30% of the cell extract without any treatment. The binding activity of NKX2-1 onto the FOXM1 promoter was evaluated through chromatin immunoprecipitation. The chromatin was isolated and immunoprecipitated with an antibody specific for NKX2-1.
