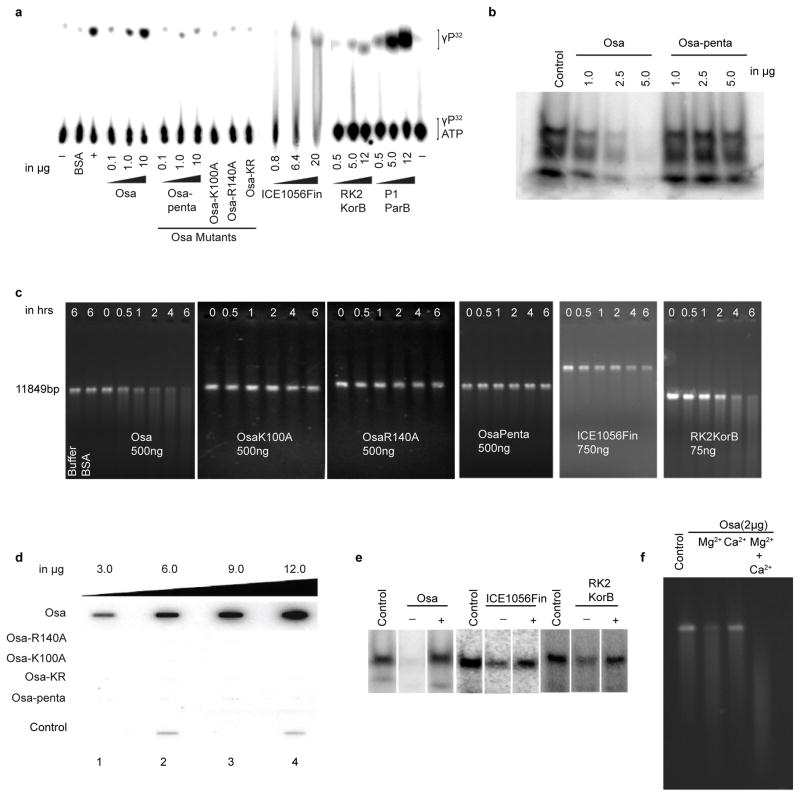
Figure 3.
The active site of Osa catalyzes both DNase and ATPase activities, and is controlled by an ATP-dependent regulatory switch. (a) ATPase activity: TLC plates were loaded with samples after ATP hydrolysis. Rv3868 of M. tb, a known ATPase, was used as a positive control. (b) DNase activity: A 90-meric ssDNA was used as a substrate. (c) DNase activity of Osa mutants and various ParB/Srx superfamily proteins using linearized pCAMBIA1301 as the substrate. (d) γ-Phosphate retention after ATP hydrolysis: slot blot overlayed with [γ-P32]ATP. The control lanes were loaded with 1: BSA, 2,4: Cdk2/Cyclin E kinase (Abcam). Slots overlayed with [α-P32]ATP did not show comparable bound radioactivity, confirming bound γPhosphate. (e) The ATP-dependent switch in Osa, ICE1056Fin and RK2 KorB: nuclease reactions were performed using labeled 90mer ssDNA substrate with 5–10 μg of proteins for 6 hrs at 37 °C and loaded onto 4–16 % TBE-PAGE. ‘−’ and ‘+’ indicate the absence and presence of 10 mM ATP in the reaction mix. (f) Metal-ion ion-dependency of Osa DNase activity. Osa shows optimal DNase activity in the presence of magnesium ion than calcium.