Abstract
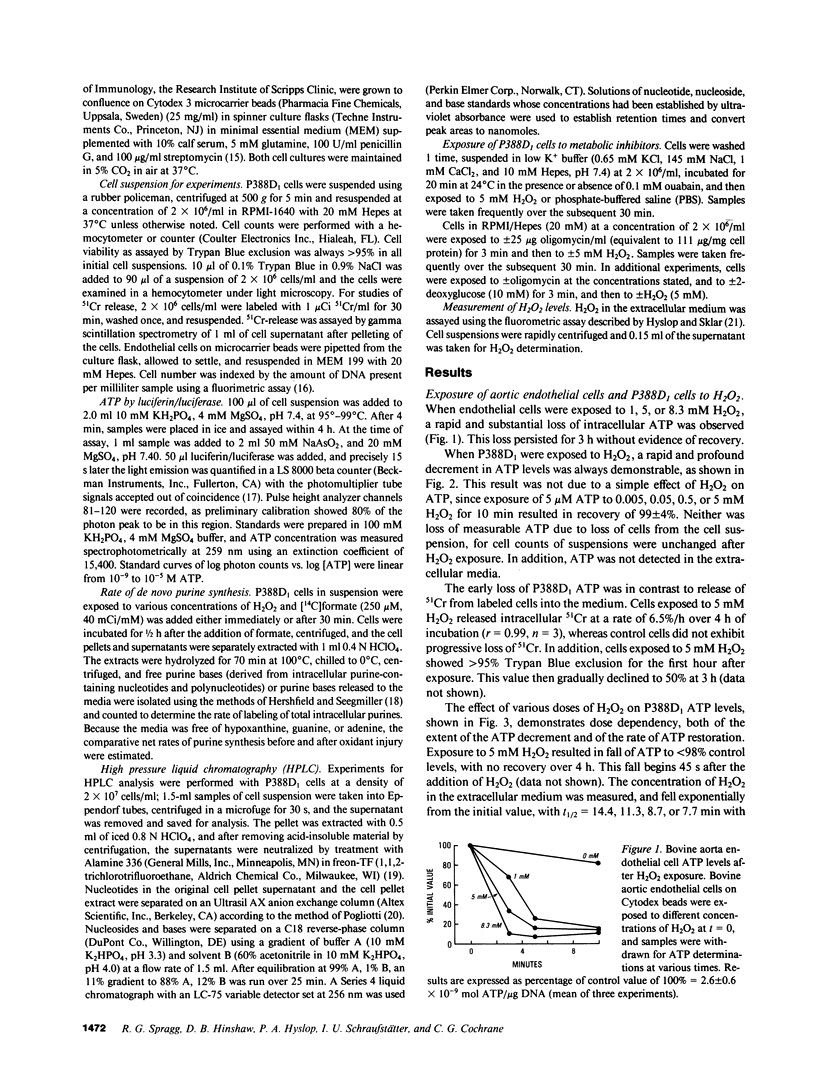
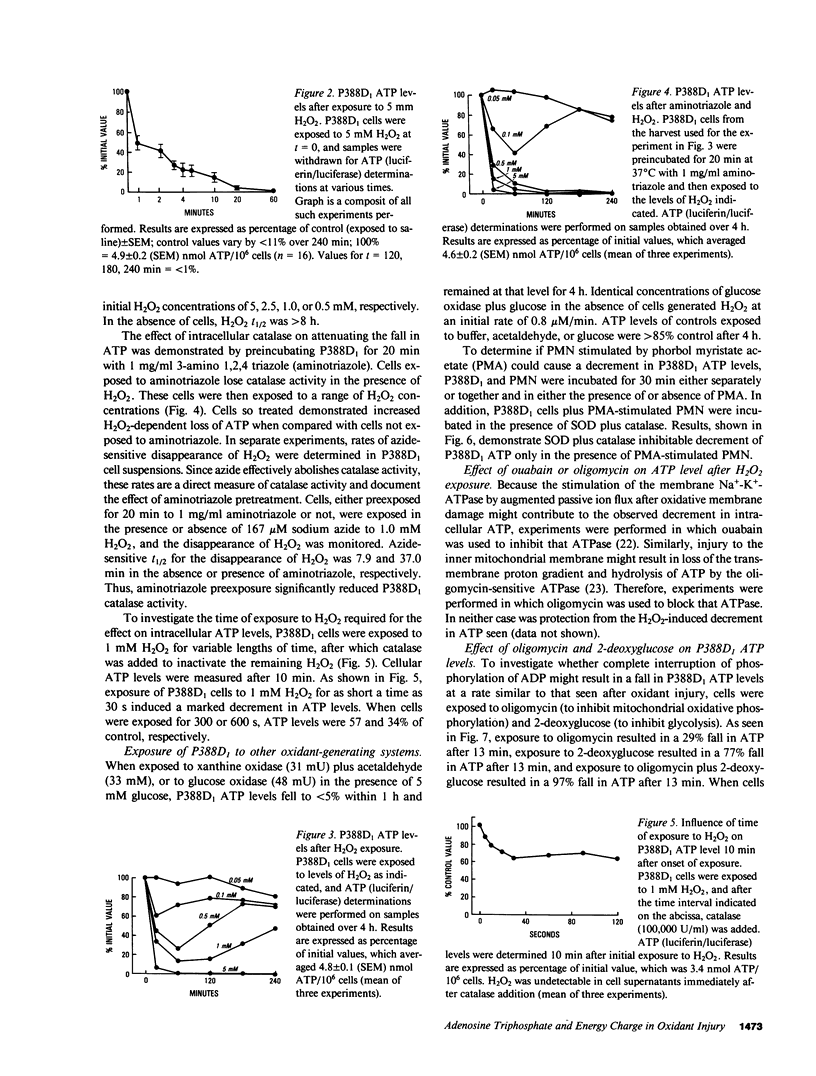
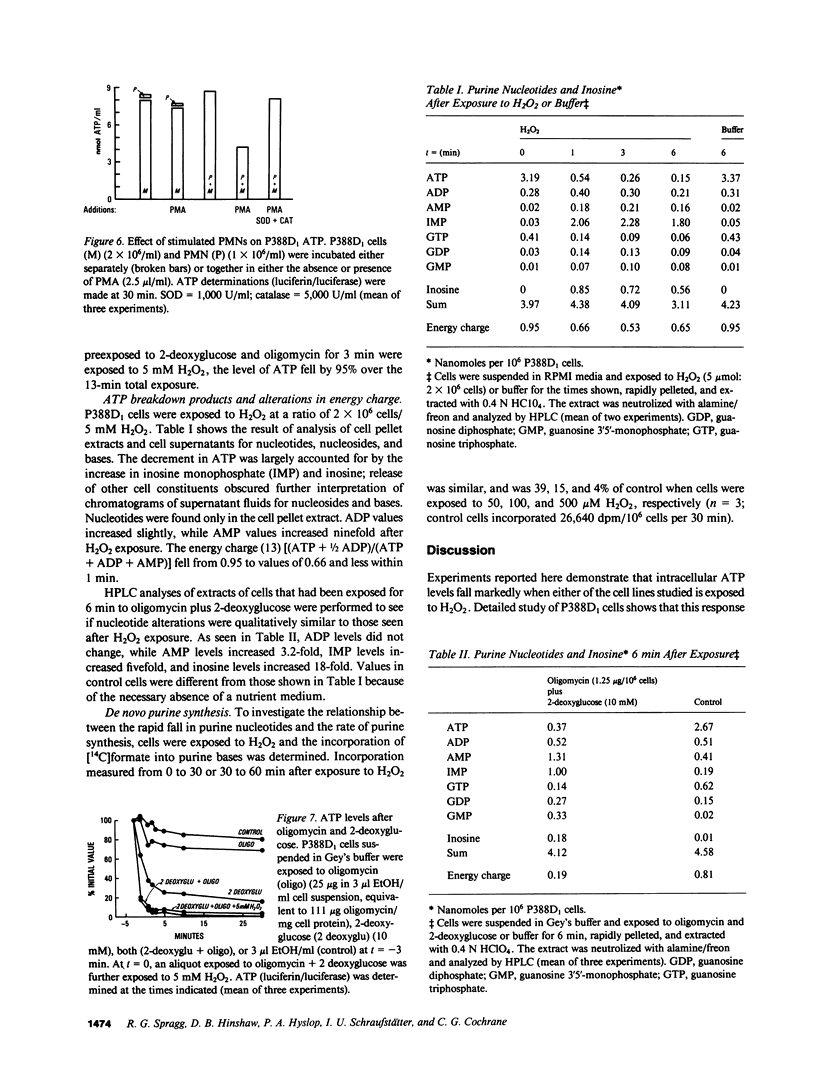
To investigate mechanisms whereby oxidant injury of cells results in cell dysfunction and death, cultured endothelial cells or P388D1 murine macrophage-like cells were exposed to oxidants including H2O2, O2-. (generated by the enzymatic oxidation of xanthine), or to stimulated polymorphonuclear leukocytes (PMN). Although Trypan Blue exclusion was not diminished before 30 min, cellular ATP was found to fall to less than 30% of control values within 3 min of exposure to 5 mM H2O2. Stimulated PMN plus P388D1 caused a 50% fall in cellular ATP levels. During the first minutes of oxidant injury, total adenylate content of cells fell by 85%. Cellular ADP increased 170%, AMP increased 900%, and an 83% loss of ATP was accompanied by a stoichiometric increase in IMP and inosine. Calculated energy charge [(ATP + 1/2 AMP)/(ATP + ADP + AMP)] fell from 0.95 to 0.66. Exposure of P388D1 to oligomycin plus 2-deoxyglucose (which inhibit oxidative and glycolytic generation of ATP, respectively) resulted in a rate of ATP fall similar to that induced by H2O2. In addition, nucleotide alterations induced by exposure to oligomycin plus 2-deoxyglucose were qualitatively similar to those induced by the oxidant. Loss of cell adenylates could not be explained by arrest of de novo purine synthesis or increased ATP consumption by the Na+-K+ ATPase or the mitochondrial F0-ATPase. These results indicate that H2O2 causes a rapid and profound fall in cellular ATP levels similar to that seen when ATP production is arrested by metabolic inhibitors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEMAN D., HEIM W. G., PYFROM H. T. Effects of 3-amino-1, 2, 4-triazole (AT) on catalase and other compounds. Am J Physiol. 1956 Jul;186(1):19–23. doi: 10.1152/ajplegacy.1956.186.1.19. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982 Sep;70(3):550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Brigelius R. Glutathione oxidation and activation of pentose phosphate cycle during hydroperoxide metabolism. A comparison of livers from fed and fasted rats. Hoppe Seylers Z Physiol Chem. 1983 Aug;364(8):989–996. doi: 10.1515/bchm2.1983.364.2.989. [DOI] [PubMed] [Google Scholar]
- Busch C., Cancilla P. A., DeBault L. E., Goldsmith J. C., Owen W. G. Use of endothelium cultured on microcarriers as a model for the microcirculation. Lab Invest. 1982 Nov;47(5):498–504. [PubMed] [Google Scholar]
- Chapman A. G., Miller A. L., Atkinson D. E. Role of the adenylate deaminase reaction in regulation of adenine nucleotide metabolism in Ehrlich ascites tumor cells. Cancer Res. 1976 Mar;36(3):1144–1150. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M., Scheffler I. E. Energy metabolism in respiration-deficient and wild type Chinese hamster fibroblasts in culture. J Cell Physiol. 1976 Sep;89(1):39–51. doi: 10.1002/jcp.1040890105. [DOI] [PubMed] [Google Scholar]
- Fisher A. B. Energy status of the rat lung after exposure to elevated PO2. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):56–59. doi: 10.1152/jappl.1978.45.1.56. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Hershfield M. S., Seegmiller J. E. Regulation of de novo purine biosynthesis in human lymphoblasts. Coordinate control of proximal (rate-determining) steps and the inosinic acid branch point. J Biol Chem. 1976 Dec 10;251(23):7348–7354. [PubMed] [Google Scholar]
- Holmsen H., Robkin L. Hydrogen peroxide lowers ATP levels in platelets without altering adenyalte energy charge and platelet function. J Biol Chem. 1977 Mar 10;252(5):1752–1757. [PubMed] [Google Scholar]
- Hyslop P. A., Sklar L. A. A quantitative fluorimetric assay for the determination of oxidant production by polymorphonuclear leukocytes: its use in the simultaneous fluorimetric assay of cellular activation processes. Anal Biochem. 1984 Aug 15;141(1):280–286. doi: 10.1016/0003-2697(84)90457-3. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Pogolotti A. L., Jr, Santi D. V. High-pressure liquid chromatography--ultraviolet analysis of intracellular nucleotides. Anal Biochem. 1982 Nov 1;126(2):335–345. doi: 10.1016/0003-2697(82)90524-3. [DOI] [PubMed] [Google Scholar]
- Rubin D. B., Housset B., Jean-Mairet Y., Junod A. F. Effects of hyperoxia on biochemical indexes of pig aortic endothelial function. In Vitro. 1983 Aug;19(8):625–634. doi: 10.1007/BF02619576. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer L. A. Control of adenosine monophosphate catabolism in mouse ascites tumor cells. Cancer Res. 1978 Apr;38(4):1057–1063. [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Test S. T., Weiss S. J. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J Biol Chem. 1984 Jan 10;259(1):399–405. [PubMed] [Google Scholar]
- Thayer W. S., Hinkle P. C. Synthesis of adenosine triphosphate by an artificially imposed electrochemical proton gradient in bovine heart submitochondrial particles. J Biol Chem. 1975 Jul 25;250(14):5330–5335. [PubMed] [Google Scholar]
- Trachtenberg M. C., Packey D. J., Sweeney T. In vivo functioning of the Na+, K+-activated ATPase. Curr Top Cell Regul. 1981;19:159–217. doi: 10.1016/b978-0-12-152819-5.50022-1. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]


