Figure 2.
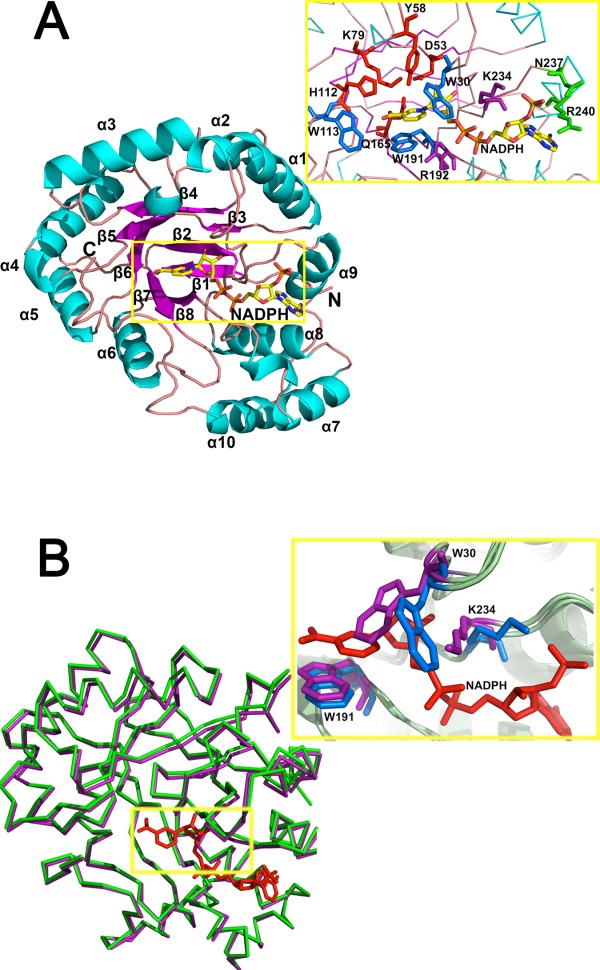
Residues involved in NADPH-binding. (A) Structural details of the (β/α)8 of AKR5C3/NADPH and key residues involved in NADPH-binding pocket. Catalytic tetrad of D53, Y58, K79, and H112 are colored in red, NADPH nicotinamide moiety recognition residues (W30, W113, and W191) are colored in blue, potential NADPH-binding residues (R192 and K234) are colored in magenta, and putative adenine moiety recognition residues (N237 and R240) are colored in green. (B) Superposition of the apoform AKR5C3 and AKR5C3/NADPH binary complex. The ribbon representation of apoform is in green, the binary complex form is in magenta, and NADPH is in red. The detailed NADPH binding-induced conformational alterations are observed in residues W30 and K234. Residues with conformational change are in marine (AKR5C3/NADPH binary complex) and purple (AKR5C3 apoform). An interactive view is available in the electronic version of the article.
