Figure 4.
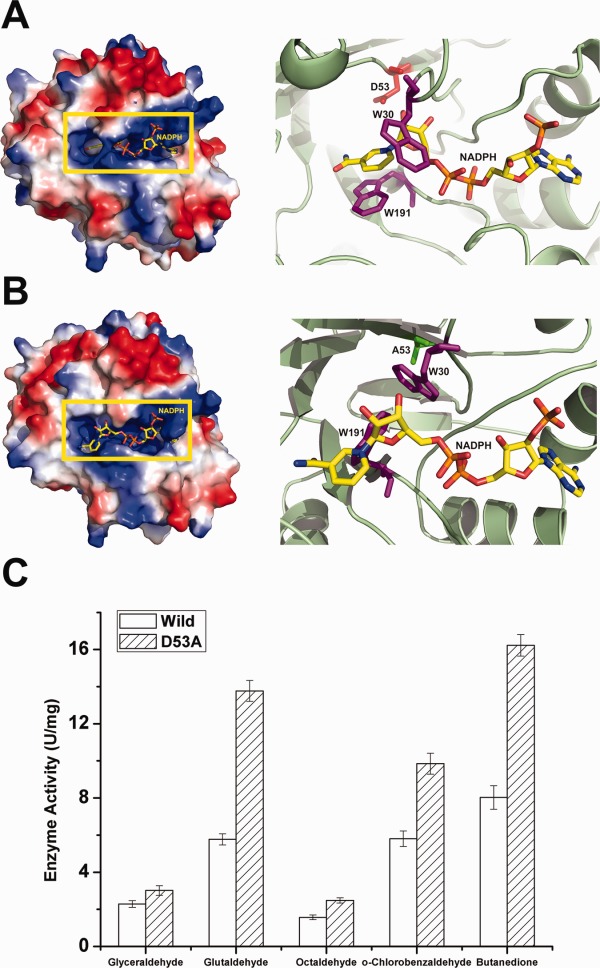
Structural observations of AKR5C3-D53A/NADPH and reduction activity assays. (A) Electrostatic potential surface view of AKR5C3. D53 is shown in red and side chains of residues W30 and W191 are shown in magenta. (B) Electrostatic potential surface view of AKR5C3-D53A. A53 is shown in green and side chains of residues W30 and W191 are shown in magenta. Compared with the corresponding residues in wild AKR5C3 binary complex, the side chains of W30 and W191 in AKR5C3-D53A/NADPH showed a rotation and the bound NADPH molecule rearranged the nicotimide moiety. (C) Enzyme activity of AKR5C3 and mutant AKR5C3-D53A. An interactive view is available in the electronic version of the article.
