Figure 5.
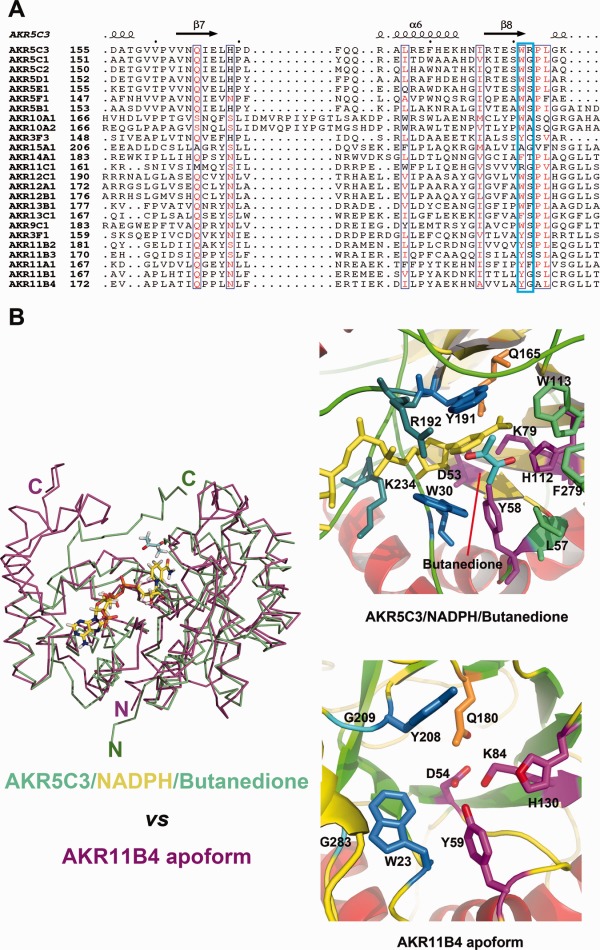
Key residues W191 and R192 involved in substrate catalysis. (A) Sequences (involved in substrate binding or recognition) alignment between AKR5C3 and other bacterial AKRs. The listed bacterial AKRs are from http://www.med.upenn.edu/akr/tree.shtml. W191 is relatively conserved while R192 is much variable. AKR11B4 and AKR5C3 shared a similar substrate spectrum but neither W191 nor R192 is conserved. (B) Structural superimposition of AKR5C3/NADPH/Butanedione (colored in green) and AKR11B4 in apoform (colored in magenta). The bound NADPH is highlighted in stick and colored in yellow. The right panel is cartoon presentation of the detailed substrate-binding pocket. The catalytic tetrad residues are colored in magenta, NADPH recognition residues are colored in blue, conserved Gln residue is colored in orange, whereas the putative aromatic cage residues for substrate binding are colored in marine. Substrate butanedione is colored in cyan.
