Figure 6.
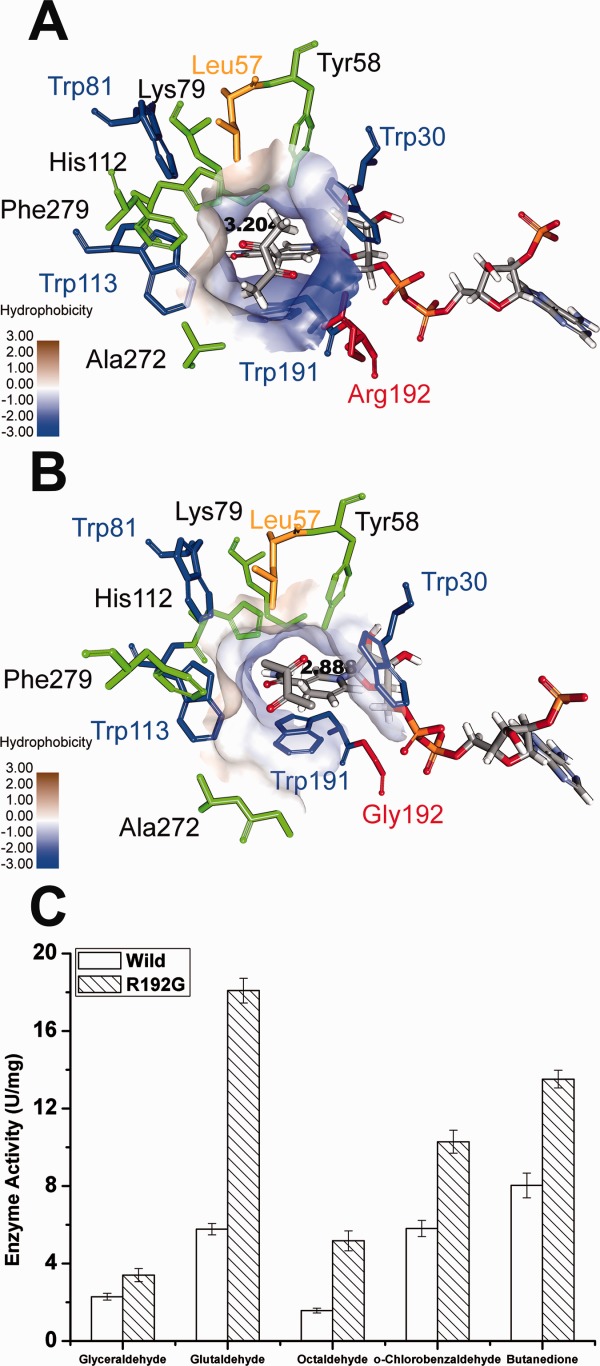
Structural insights into the AKR5C3-R192G/NADPH/ butanedione model and the improved reductive activity of AKR5C3-R192G. (A) The substrate-binding pocket was putatively identified upon butanedione docking in AKR5C3/NADPH modeled structure. (B) The identical view of (A) is shown with AKR5C3-R192G/NADPH/ butanedione model. The residues involved in the substrate-binding pocket were shown and marked. Hydrophobicity surface of substrate-binding pocket was demonstrated respectively in AKR5C3/NADPH crystal structure (A) and AKR5C3-R192G/NADPH (B). (C) Enzyme activity of AKR5C3-R192G.
