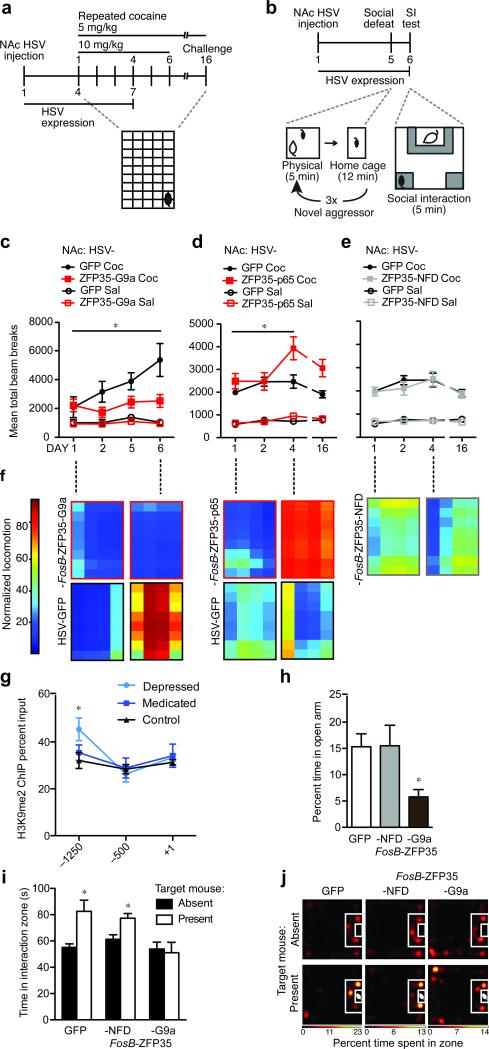
Figure 4.
Engineered transcription factors bidirectionally modulate cocaine- and stress-evoked behaviors. (a) Locomotor activity was assessed during repeated cocaine exposure in mice injected intra-NAc with HSV-FosB-ZFP35-G9a [n=7], -p65 [n=9], -NFD [n=9] or control virus [n=5 (10mg/kg) or n=10 (5mg/kg)]. (b) Social behavior was measured 24 hours after subthreshold social defeat in mice injected with HSV-FosB-ZFP35-G9a or control virus into the NAc. (c) At high doses of cocaine (10 mg/kg), locomotor behavior sensitizes over time and this effect was blocked by HSV-FosB-ZFP35-G9a in NAc. Repeated measures ANOVA revealed an interaction between day, cocaine, and virus [F(3,18)=4.00, *P=0.024] on locomotor behavior. Among cocaine treated mice, there is a main effect of virus [F(1,10)=8.81, *P=0.026] and a trend for an interaction between virus and day [F(2,8)=3.33, τP=0.077] such that GFP locomotor behavior is enhanced above FosB-ZFP35-G9a levels by treatment day 6 [t(1,10)=2.61, *P =0.026]. (d) At low doses (5 mg/kg), cocaine-induced locomotor behavior sensitizes over time with FosB-ZFP35-p65 in NAc [repeated measures: interaction between day, treatment, and virus [F(3,32)=4.71, *P=0.008]. Among cocaine treated mice, there is an interaction between virus and day [F(2,15)=7.08, *P=0.003] and a trend for a main effect of day among FosB-ZFP35-p65 [F(1,32)=2.85, τP=0.053] but not GFP cocaine treated animals. Among cocaine-treated animals, FosB-ZFP35-p65 enhances locomotor behavior above GFP levels by treatment day 4 [t17=2.58, *P=0.020] through day 16 [t17=2.92, *P=0.009]. (e) NAc injection of HSV-FosB-ZFP35-NFD, like controls, did not display cocaine locomotor sensitization to a low dose of cocaine. Repeated measures failed to find an interaction among day, treatment, and virus [F(1,28)=0.13, P=0.944]. There is no effect of day among cocaine-treated mice [F(1,28)=0.86, P=0.471]. HSV-GFP data are the same as in (Fig. 4d). (f) Heat maps show representative locomotor data within the chamber for mice over the course of repeated cocaine exposure. (g) H3K9me2 is significantly enriched at –1250 bp from the FosB TSS in depressed humans [t22=2.19, p=0.040, n=8, 17] compared to levels in control subjects. (h) FosB-ZFP35-G9a in the NAc reduced exploration of the open arm in the elevated plus maze, compared to control virus [t12=2.36, *P=0.036; n=7]. (i) HSV-FosB-ZFP35-G9a in the NAc blocked increased exploration of a novel aggressor mouse after exposure to subthreshold social defeat, compared to control virus [t123.1, *P=0.009; n=7] with no effect of HSV-FosB-ZFP35-NFD [t12=3.2, *P=0.008; n=7]. (j) Representative heatmaps of social interaction after a subthreshold defeat stress show a preference for the interaction zone when a target mouse is present for mice injected with control virus and HSV-FosB-ZFP35-NFD, but not HSV-FosB-ZFP35-G9a.