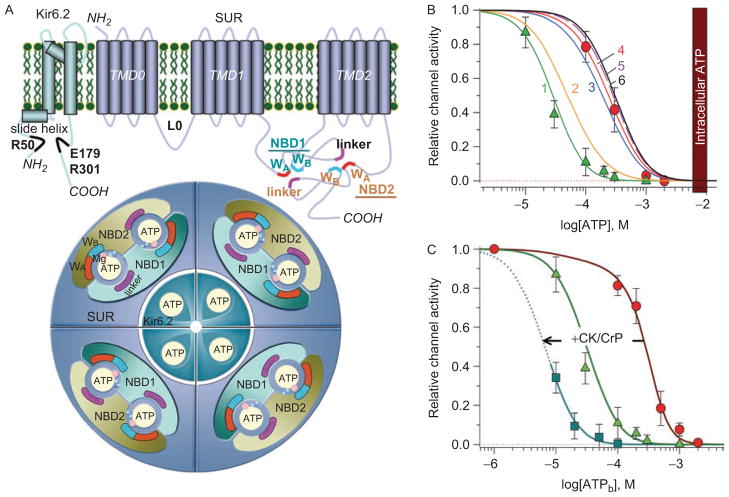
Figure 1.
Structure and nucleotide-dependent gating of KATP channel complexes. (A) Kir6.2 possesses two transmembrane (TM) pore-forming α-helices and a slide helix, which by lateral movement contributes to the gating mechanism. The putative ATP binding residues are indicated in black. SUR2A is comprised of three bundles of transmembrane-spanning domains and two cytosolic ATP-binding cassettes (ABC), i.e. nucleotide binding domains (NBD1 and NBD2). WA and WB denote the Walker A and Walker B motifs conserved within ABC proteins that, along with the linker regions, are critical for coordination of nucleotides within binding pockets. Each ATP molecule is sandwiched between the Walker motifs of one NBD and the linker region from another NBD. The whole KATP channel complex is formed by four SUR co-assembled with four Kir6.2 subunits, harboring 12 nucleotide binding sites in total. Cofactors of the hydrolytic reaction, Mg2+ (pink) and water molecules (blue), are also indicated. (B) MgADP antagonizes ATP-induced inhibition of cardiac KATP channels. In excised patches from isolated cardiomyocytes, the ATP sensitivity of KATP channels is defined by an IC50 of 27 ± 5 μM in the absence (triangles) versus 270 ± 19 μM in the presence (circles) of 100 μM ADP. At increasing ADP concentrations (curve 1: 0; 2: 10; 3: 50; 4: 100; 5: 500; 6: 1000 μM), following suturation of all binding sites no further reduction in ATP sensitivity of KATP channels can be achieved (adapted from Selivanov et al., 2004). The vertical bar corresponds to 6–10 mM intracellular ATP levels. (C) Concentration–response curves defining ATP-induced KATP channel inhibition in open cell-attached patches, where KATP channels operate in a relatively intact cellular environment, in the absence (circles) and presence (squares) of 1 mM creatine phosphate (CrP). Intracellular ATPase activities define the right-shift of the concentration-response curve (IC50 ~300 μM) in the open-cell attached configuration, relative to the values measured in excised patches (triangles), and can be effectively left-shifted to an IC50 ~10 μM by activation of the ATP-regenerating endogenous creatine kinase (CK) when the reaction substrate (CrP) is applied. By itself, CrP has no significant effect on KATP channel activity (Abraham et al., 2002).