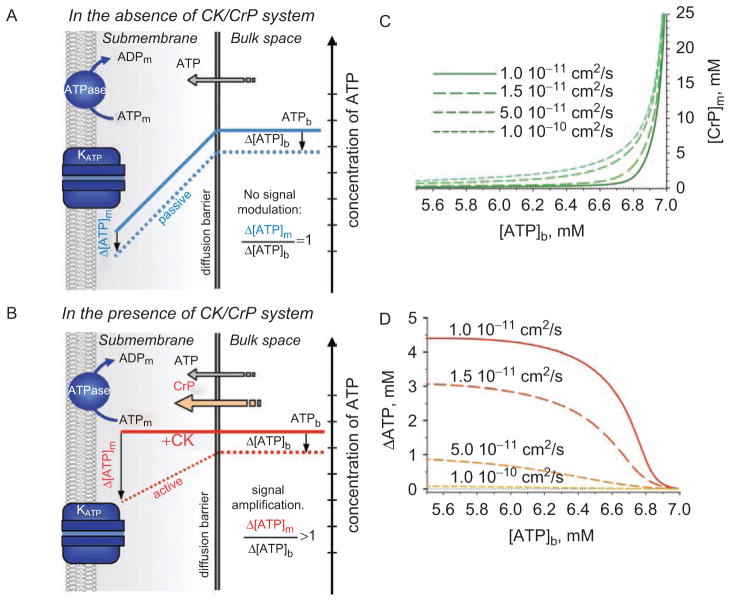
Figure 2.
Nucleotide signals at a strong diffusion barrier secluding submembrane space. (A) In the absence of the creatine kinase/creatine phosphate (CK/CrP) phosphotransfer system, local ATPase activities set a nucleotide gradient between cellular compartments (blue solid line). A drop in bulk ATP levels (Δ[ATP]b) is passively followed by changes in submembrane concentrations (Δ[ATP]m), i.e. the gradient remains unchanged (blue dotted line) with no signal modulation. (B) In the presence of CK/CrP system, the facilitated diffusion catalyzed by CK virtually voids the concentration gradient (red solid line), but actively modulates (i.e. amplifies) changes in submembrane nucleotide content (Δ[ATP]m, red dotted line) that exceed the deviation of bulk ATP. (C and D) Submembrane concentrations of creatine phosphate ([CrP]m) and ATP gradients (ΔATP) between bulk and submembrane space, respectively, calculated at the different values of apparent diffusion coefficients (system of equations 13, Selivanov et al., 2004) following a drop of bulk ATP ([ATP]b). Calculations were performed with 7 mM total nucleotide content; 40 mM total Cr/CrP level; sarcolemmal ATPase flux 4.7·10−6 μmol/cm2/s; Δx = 0.2 μm and KCK=160.