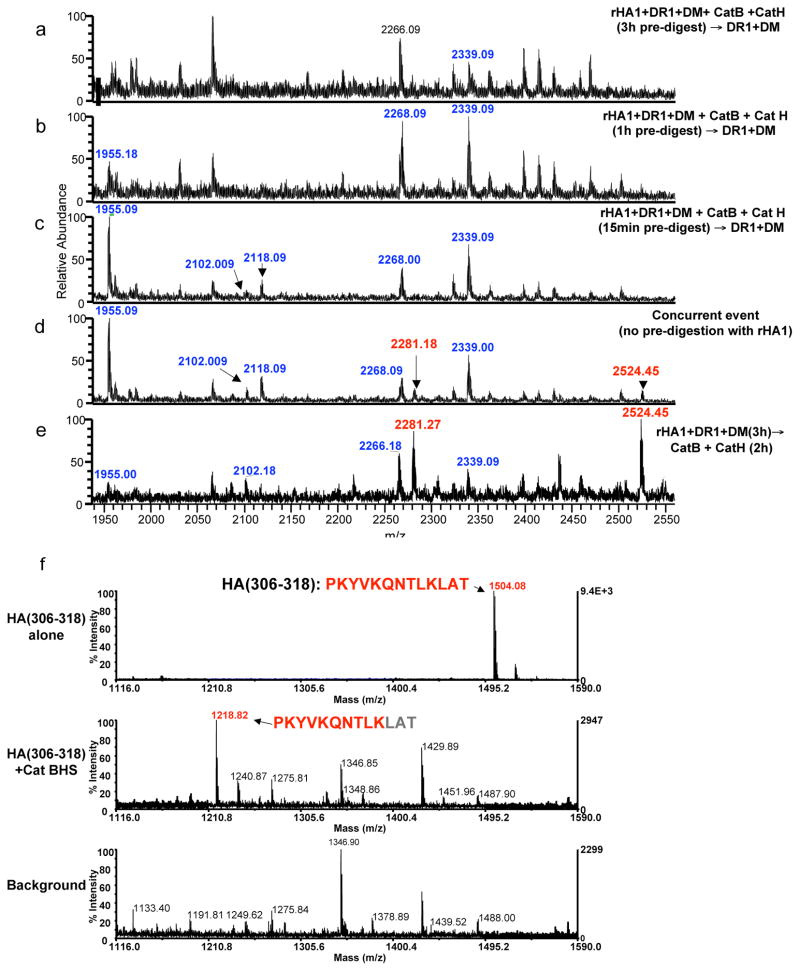
Figure 4. Degradation of HA(306–318) by the cathepsins outcompetes its capture by HLA-DR1.
(a–e) The mass spectra of peptides eluted from DR1. Mass species in red represent rHA1 fragments containing the DR1 restricted immunodominant HA(306–318) epitope. Peptides highlighted in blue represent other rHA1-derived peptides eluted from DR1. Recombinant HA1 was first exposed to cathepsins B and H for (a) 3h, (b) 1h, or (c) 15 minutes, followed by incubation with DR1 and DM for 2 h at 37°C. (d) Mass spectrum of peptides eluted from DR1 when all components were mixed simultaneously and incubated together for 3h. (e) Mass spectra of peptides eluted from DR1 when rHA1 was incubated with DR1 and DM for 3h first, and then incubated with the cathepsins for additional 2h. (f) Sensitivity of synthetic HA(306–318) to the cathepsins tested by direct exposure to cathepsins B, H, and S. Top spectrum shows HA(306–318) peptide alone. Middle spectrum is the sample reaction containing HA(306–318) incubated with the cathepsins for 1h at 37°C, and the bottom spectrum shows the background sample containing the cathepsin mix without the synthetic peptide. Experiments were repeated three times.