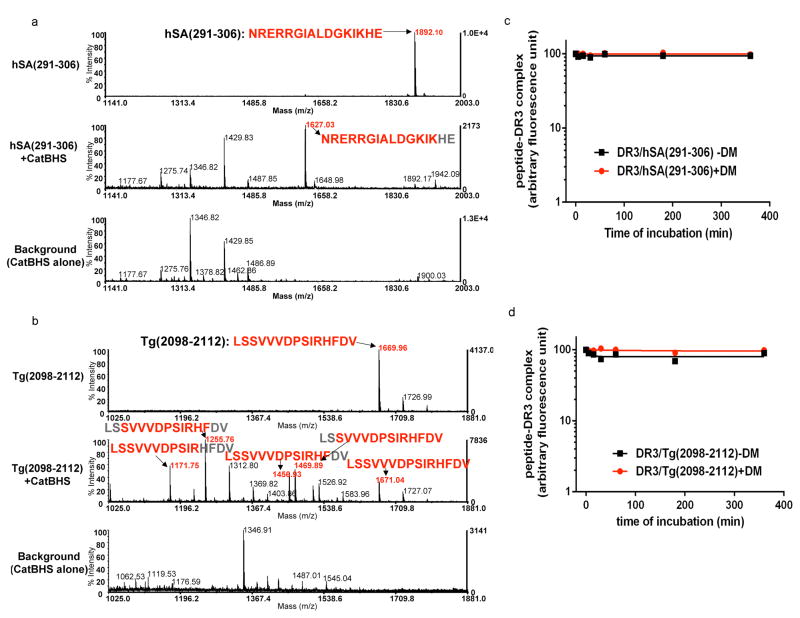
Figure 7. HLA-DR3 binding core region of autoantigen derived immunodominant epitopes show resistance to the cathepsins and HLA-DM.
(a–b) Mass spectra of retinal arrestin derived dominant epitope, hSA(291–306), and thyroglobulin-derived dominant epitope, Tg(2098–2112), after digestion with the cathepsins. Untreated synthetic peptides (a) hSA(291–306) or (b) Tg(2098–2112) are shown on the top spectra in a and b. Peptides were incubated with cathepsin B, H, and S for 1h at 37°C (shown in the middle spectra). Background controls that included cathepsins alone are shown in the bottom spectra. The experiments were repeated twice.
(c–d) Dissociation kinetics of fluorescently labeled hSA(291–306) or Tg(2098–2112) from DR3. (c) FL-hSA(291–306)/DR3 or (d) FL-Tg(2098–2112)/DR3 complexes were formed over a three day incubation with peptides in 37°C and the unbound peptides were removed by spin column separation. Dissociation experiments were in the presence of 100μM unlabeled hSA(291–306) or 100μM unlabeled Tg(2098–2112) peptides at 37°C for the indicated time in the presence (circle) or absence (square) of DM. The fluorescence of the labeled complex before dissociation was arbitrarily assigned a value of 100.0, and fluorescence after dissociation is expressed as a fraction of fluorescence before dissociation. Dissociation experiments were repeated twice.