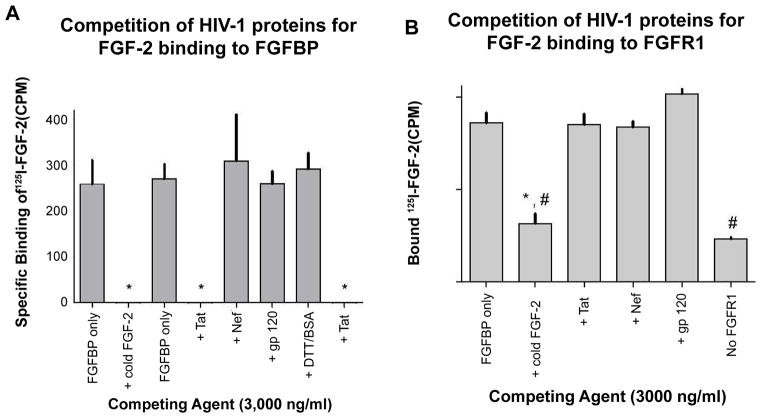
Figure 3. Effect of HIV-Tat protein on FGF-2 binding to BP1 (A) or FGF receptor (B) in cell-free binding assays with recombinant proteins.
A. Immobilized recombinant human BP1 protein was incubated with [125]-I-FGF-2 in the absence (control) or presence of different competitors: FGF-2, HIV-Tat, Nef and gp120. Data points represent specific binding of [125]-I-FGF-2 after subtraction of binding to wells coated with blocking solution only. Competition for [125]-I-FGF-2 binding was only observed with cold FGF-2 or HIV Tat protein as competitors. *, p<0.05 relative to “FGFBP only”.
B. An FGF receptor-IgG fusion protein (500 ng/ml; FGFR1) and [125]-I-FGF-2 (20 ng/ml) were incubated with protein A/G agarose beads, in the absence (control) or presence of cold FGF-2, Tat, Nef, or gp120. One sample set with the receptor protein and radiolabeled FGF-2 was also run without the FGFR-IgG fusion protein (“No FGFR1”). Washed beads were collected and bound radioactivity was detected and is expressed relative to control. Bars are means of triplicates + SEM from one representative experiment. This experiment was repeated twice. There was no significant difference in receptor binding of [125]-I-FGF-2 in control samples versus those containing Tat, Nef, or gp 120. In the absence of FGF receptor protein or with added cold FGF-2 significantly less ligand binding was observed. *, p<0.05 relative to “FGFR1 only”; #, no significant difference between “+Cold FGF-2” and “No FGFR1”.