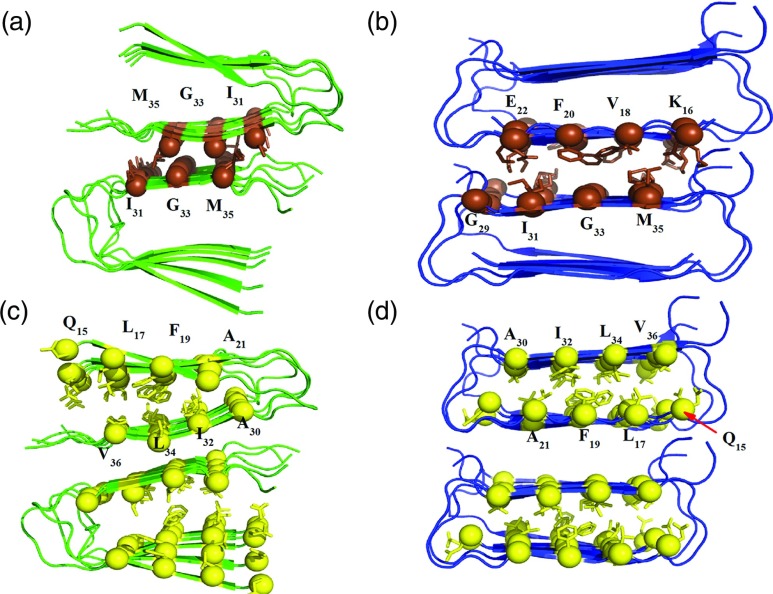
FIG. 3.
Face-to-face interactions in decamers of Aβ wild type and Iowa mutant. The side-chains involved in the complementary interactions are shown as spheres, with yellow spheres representing the face-to-face interactions between β-sheets of the single fold residues and brown spheres representing C–C terminal side chain interactions between protein layers. Parallel systems are shown in green and antiparallel is shown in blue. (a) Side chain interactions along the C-terminal β-sheets interface residues I31/M35, G33/G33, and M35/I31 for the parallel β-sheet double fold; and (b) side chain interactions along the C-terminal β-sheets interface residues G29/E22, I31/F20, G33/V18, and M35/K16 for the antiparallel β-sheet double fold. Face-to-face interactions within β-sheets between the single fold residues Q15/V36, L17/L34, F19/I32, and A21/A30 are shown in (c) for the wild-type parallel β-sheet, and in (d) for the antiparallel β-sheet in the Iowa mutant.