Abstract
[Purpose]
Pine needle is a kind of medicinal plant ingested traditionally for a variety of purposes. Therefore, we examined the antioxidant and antiapoptotic capacities of pine needle ingestion in high cholesterol-fed and endurance exercise-trained rats.
[Methods]
Animals were divided into six groups as; CON: normal diet control group; EX: normal diet and exercise training group; HC: high cholesterol diet group; HCE: high cholesterol diet and exercise training group; HCP: high cholesterol and pine needle group; HCPE: high-cholesterol and pine needle diet with exercise training group, respectively. Each group consisted of seven Sprague-Dawley male rats. The swim-training groups, EX, HCE, and HCPE swam in the swim pool 60 min/d and 5 d/week for 5 weeks. During the rearing periods, freeze-dried pine needle powder mix with 5% of the high cholesterol diet was supplied to the HCP and HCPE groups. Gastrocnemius muscle was used as the skeletal muscle. Malondialdehyde (MDA), Mn-containing superoxide dismutase (Mn-SOD), Cu, Zn containing superoxide dismutase (Cu,Zn-SOD), and glutathione peroxidase (GPx) were analyzed for their antioxidant capacities. Finally, p53, Bcl-2 (B-cell lymphoma 2), caspase-3 protein expression was analyzed to determine antiapoptotic ability.
[Results]
MDA showed low content in HCPE compared to the HC. Mn-SOD, Cu,Zn-SOD, and GPx protein expression was significantly increased by pine needle ingestion and/or exercise training. In addition, suppression of p53 protein expression resulted in Bcl-2 increase followed by caspase-3 decrease with/without pine needle ingestion and exercise training.
[Conclusion]
When exercise training in addition to pine needle powder ingestion may be a helpful nutritional regimen to athletes and exercisers.
Keywords: pine needle, antioxidant, antiapoptosis, endurance exercise, rat
INTRODUCTION
High fat and high cholesterol diets increase weight, blood lipid and cholesterol concentration [1]. It is known that the increased internal lipid damages proteins and cells as well as DNA by converting reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide, and hydroxyl radical into malondialdehyde (MDA) [2]. It is also known that the formation of adipocyte molecules inhibits the balance of pro-apoptotic and anti-apoptotic factors, and consequently leads to the increase of apoptosis resistance and causes diseases associated with obesity [3,4].
The appropriate exercise in conjunction with dietary treatment for obesity prevention is recommended as a very important method for preventing metabolic syndrome. However, oxygen consumption during exercise increases up to 10-15 times, and the oxygen supply to muscle tissues is dramatically increased. Also, oxidation generation by the electron transport system increases [5,6]. The increase of ROS accelerates cell apoptosis by impairing oxidation in the mitochondrial membrane and reducing DNA damage of lymphocytes [7-9]. Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) are the endogenous antioxidant enzymes that remove such ROS, but because there are situations where their generation and action abilities are exceeded, exogenous antioxidants are required. Antioxidants significantly contribute to the antioxidant defense, but because synthesis in the body is impossible, these antioxidants must be necessarily taken in as a food resources [10,11]. Therefore, for the improvement of chronic diseases and health maintenance, food selection is very important.
Recently, the availability of suitable foods that provide antioxidants has increased. Among the available options, medicinal plants are generally considered to be safe substitutes [12,13]. Proven functions of medicinal plants include anti-cancer [14], anti-obesity [15], cholesterol reduction and antidiabetic effects [16]. In particular, it has been reported that the needles of a pine tree which accounts for about 40% of the mountainous area in Korea contain essential oil components such as α-oinene, β-pinene and camphene, and flavonoids, pinitol and chlorophyll, vitamin A, vitamin C, protein, fat, phosphorous, iron, enzymes and minerals which are typical for medicinal plants [17,18]. These pine needles have been reported to demonstrate serum lipid lowering [19], anti-obesity [20], anti-oxidation [21], and anti-inflammation activities [22]. The additional intake of 5% pine needle powder of the total recommended dietary values lowered triglyceride and high density lipoprotein (HDL-C) concentrations. The amount of fatty liver and fatty cells caused by high cholesterol or a high-fat diet were also reduced, and the expression of PPAR-γ. mRNA in adipocytes cell was inhibited. A lower pine needle intake of 2-4% reduced the abdominal fat [18-20]. It has also been reported that in in vivo experiments, pine needles influence the concentration of Cu,Zn-SOD, and have anti-tumor, anti-mutant, anti-cancer, anti-inflammation, anti-atherosclerosis effects [22-25].
In recent years, due to the increased interest in cholesterol intake and the reduction of cholesterol, it was determined that studies on the effects of pine needle powder and exercise on anti-oxidative enzymes and cell death when combined with high-cholesterol diets are required. Therefore, in this study, the authors intended to investigate the mechanism of mutual action for the antioxidant enzyme system and cell death by using pine needle powder which is relatively easy and cheap to obtain and serves as a functional food.
METHODS
Animal care
In this study, 42 four-weeks-old male SD rats received from Hyochang Science in Daegu were used. The rats were bred individually, and the internal environment of the breeding room was 23-25℃. The relative humidity was maintained at around 60% and the light and dark period was maintained at 12 hour intervals from 08:00 to 20:00. The groups were divided into the control diet (CON) group, control diet + exercise (EX) group, high-cholesterol diet + exercise (HCE) group, high-cholesterol diet + pine needle (HCP) group, and high-cholesterol diet + pine needle + exercise (HCPE) group.
Dietary composition
The pine needles were prepared based on AIN-76 dietary composition, by processing pine needles collected in Korea to powder after freeze-drying. The pine needle powder was prepared by adding 5% of the designated diet [18]. The rats took in the food in 10 g amounts supplied at 8:00 AM and 8:00 PM for an hour, and they could freely drink water.
Exercise training
Exercise was performed for 5 weeks when the rats turned 6-weeks-old after pre-breeding. The swimming exercise was performed 5 times a week with the water temperature maintained at 35.0 ± 1.0℃ in a water tank (30 cm width × 30 cm length × 80 cm depth) and space secured to enable each rat sufficient space to swim. At first, the exercise lasted for 10 minutes, and was gradually increased every day. By the third week, swimming exercise lasted for 60 minutes after adaptation training.
Sampling
The samples were quantified by extracting and separating the gastrocnemius after anesthetizing using a small animal portable anesthesia system. The samples were extracted after fasting for 12 hours, and were stored in the freezer at -80℃ until analysis after stopping muscle activity in liquid nitrogen.
Analysis
Muscle MDA
0.5 g of muscle was homogenized with 5 ml HEPES buffer(0.25 M sucrose, 0.5 mM EDTA, 5 mM HEPES) by measuring the MDA concentration of muscle. The above sample was used for analysis after separating 2 ml of the supernatant liquid by centrifugation at 6,500 rpm for 20 minutes. After mixing 500 μl of the sample separated for the MDA concentration analysis with 2.5 ml of 10% TCA buffer, the mixture was left at room temperature for 10 minutes. The mixture was then separated at 3,500 rpm for 10 minutes using a centrifugal separator. The supernatant was discarded and the lower layer was mixed with 2.5 ml of 0.05 M H2SO4 and 3 ml of TBA buffer. This mixture was boiled in a water bath for 30 minutes at 95℃ and cooled by removing it from the water bath and leaving it at room temperature. Then 3 ml of the buffer mixed in a ratio of n-butanol:pyridine (15:1) was added, and the mixture was separated by centrifugation at 3,000 rpm after mixing for 90 minutes. MDA was measured at the wavelength of 530 nm by UV-Spectrometer (Optizen POP, Korea) using the supernatant of the sample.
Western blotting
The protein quantification was measured at the absorbance of 595 nm by placing each 200 μl sample in an ELISA plate (TECAN, Austria) after completely mixing 1 μl of the upper layer with 1 ml brad-ford of the sample homogenized in the HEPES buffer. For the treatment of muscle tissue for SOD analysis, the homogenization was carried out by adding RIPA buffer to the sample. The sample was separated by a centrifuge at 1200 g for 10 minutes, after which the homogenization was finished. The supernatant liquid was then separated, and the absorbance at 595 nm was measured by placing 200 μl of each sample in an ELISA (TECAN, Austria) plate after completely mixing 1 μl of the supernatant with 1 ml brad-ford of the samples from the supernatants separated for protein quantification. The samples were quantified by mixing with Laemmli sample buffer. In order to analyze the expression level of protein, the protein was separated by electrophoresis for 1 hour at 100 V after loading 20 μl of each protein sample in 10% sodium dodecyl sulphate (SDS)-polyacrylamide gel. The separated protein was then transferred to polyvinylidene difluoride membranes. Blocking buffer (TBST buffer; 50 mM Tris-HCl, 150 mM NaCl, 0.05%, Tween 20) containing 5% BAS was applied for 1 1/2 hours after the transition was complete. Mn-SOD (Santa cruz, sc-18504, USA), p53 (abcam, ab26, UK), Bcl-2 (abcam, ab18210, UK), and caspase-3 (Cell Signaling, #9665, Swiss), which are the primary antibodies, were diluted according to the manufacturers’ instructions. The above primary antibodies were added over-night, after which they were washed 5 times for 10 minutes with TBST (5% tween-20). Secondary antibodies were diluted according to the manufacturers’ instructions and were added for 1 hour before washing 5 times for 10 minutes with TBST (5% tween-20). Identification of the resulting band was developed on X-ray film after illuminating a membrane using an enhanced chemiluminescene (ECL) kit. The density of the developed band was calculated by band/β-actin using the image j software.
Immunoprecipitation (IP)
For immunoprecipitation pre-treatment using the sample with protein quantification, homogenization was performed after adding 0.5 M EDTA (Duksan, Korea), lysis buffer (Gendepot, R4200-100, USA), and phosphatase inhibitor 100 × (Gendepot, P3200-001, USA) to the sample. After adding 200 μg/ml each of Cu-Zn-SOD and GPx, which are the primary antibodies, into the homogenized sample of 1ml, the mixture was left over-night (12 hours) at 4℃. After inserting 30 μl of Beads (Dynabeads, Protein A) and 1 × PBS of 500 μl in the sample left over-night and incubating at 4℃ for 1 hour, the incubated sample was washed 3 times for 5 minutes with 1 × PBS using a magnetic particle concentrator. 1 × PBS of 50 μl was added to the washed sample, and the samples were quantified by mixing with Laemmli sample buffer. Western blotting for the Cu,Zn-SOD (Santa cruz, sc-11407, USA) and GPx (Santa cruz, sc-22145, USA), which are primary antibodies, and their secondary antibodies were performed according to the manufacturers’ instructions. Identification of the band was developed on X-ray film after illuminating a membrane using an enhanced chemiluminescene (ECL) kit. The density of the developed band was calculated by band/β-actin using image j software.
Data processing
The statistical program SPSS/PC + 21.0 for Windows was used as the data analysis program for research results. All experimental results were represented as the mean and standard deviation, and one-way analysis of variance (ANOVA) was performed in order to verify the significance of each group. For the items where significant differences were indicated, post-hoc comparison was performed using the least significant difference (LSD) method. Statistically significant difference was set at p < 0.05.
RESULTS
Change of MDA (mmol/mg) content
The change of muscle MDA content is the same as shown in Fig. 1. HCPE showed a statistically significantly low value at 7.55 ± 0.21 in comparison with CON 8.57 ± 1.45, EX 8.75 ± 0.25, HCE 9.55 ± 0.20, and HCP 9.30 ± 0.31. HC showed a statistically significantly high content compared to each group at 12.17 ± 0.23 (p < 0.05).
Fig. 1.
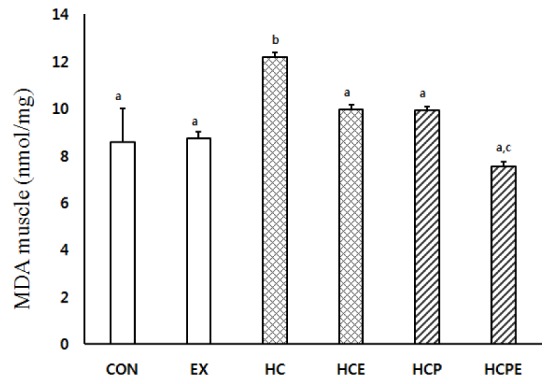
Difference of gastrocnemius muscle MDA concentrations. CON: normal diet control group; EX: normal diet and exercise training group; HC: high-cholesterol diet group; HCE: high-cholesterol diet and exercise training group; HCP: high-cholesterol and Pine Needle group; HCPE: high-cholesterol and pine needle diet and exercise training group; different letter means significance at p < 0.05, respectively.
Expression of antioxidant protein (arbitrary unit)
Expression of the Mn-SOD protein in the muscle was significantly lower for HCPE at 7.55 ± 0.21 in comparison with CON 8.57 ± 1.45, EX 8.75 ± 0.25, HCE 9.55 ± 0.20, and HCP 9.30 ± 0.31. HC displayed significantly high expression compared to the other groups at 12.17 ± 0.23 (p < 0.05; Fig. 2A). For expression of the Cu,Zn-SOD protein within muscle cells, HC 0.79 ± 0.10, HCE 1.01 ± 0.02, HCP 0.93 ± 0.05, and HCPE 1.25 ± 0.15 groups were significantly higher compared to CON 0.37 ± 0.19, and EX 0.71 ± 0.13 (p < 0.05). HCPE showed significantly higher results in comparison with EX and HC. In particular, the combination of pine needle powder intake with exercise showed an increase in muscle Cu,Zn-SOD activity (p < 0.05; Fig. 2B). In the expression of muscle GPx protein, HCE 0.90 ± 0.01, HCP 0.87 ± 0.00, and HCPE 1.14 ± 0.00 were significantly higher than CON 0.66 ± 0.00, EX 0.50 ± 0.00, while HC 0.51 ± 0.01 (p < 0.05), and EX and HC were significantly lower than each group (p < 0.05). In addition, the HCPE group showed significantly high results for both tests. The combination of pine needle powder intake with exercise showed a particularly high increase in muscle GPx activity (p < 0.05; Fig. 2C).
Fig. 2.
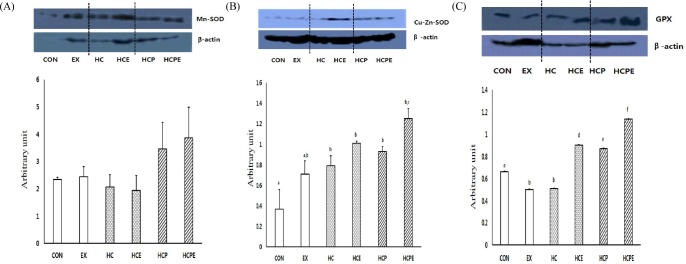
Difference of antioxidant activity protein expressions. A: Mn-SOD expression in gastrocnemius muscle, B: Cu, Zn-SOD expression in gastrocnemius muscle, C: GPx expression in gastrocnemius muscle. CON: normal diet control group; EX: normal diet and exercise training group; HC: high-cholesterol diet group; HCE: high-cholesterol diet and exercise training group; HCP: high-cholesterol and Pine Needle group; HCPE: high-cholesterol and pine needle diet and exercise training group; different letter means significance at p < 0.05, respectively.
Expression of antiapoptotic protein (arbitrary unit)
The expression level of p53 protein, a cell death factor, showed significant difference in each group, with CON 1.28 ± 0.00, EX 0.89 ± 0.00, HC 0.86 ± 0.03, HCE 1.33 ± 0.01, HCP 1.50 ± 0.01, and HCPE 0.95 ± 0.00 (p < 0.05). In particular, HCPE showed significantly lower results, so it was shown that the combination of pine needles powder intake with exercise inhibits the activity of p53 within muscle (p < 0.05; Fig. 3A). The expression level of Bcl-2, an antiapoptotic marker that inhibits the activity of p53, was significantly higher than CON 0.33 ± 0.07, with EX group 0.87 ± 0.14, HC 0.87 ± 0.13, HCE 1.04 ± 0.07, HCP 0.84 ± 0.08, and HCPE 0.95 ± 0.08 (p < 0.05; Fig. 3B). The expression level of caspase-3 protein did not show significant difference, with CON 0.88 ± 0.33, EX 0.93 ± 0.29, HC 0.62 ± 0.24 HCE 0.48 ± 0.12, HCP 0.92 ± 0.36, and HCPE 0.47 ± 0.22. However, HCE and HCPE showed a tendency to decrease in comparison with other groups (Fig. 3C).
Fig. 3.
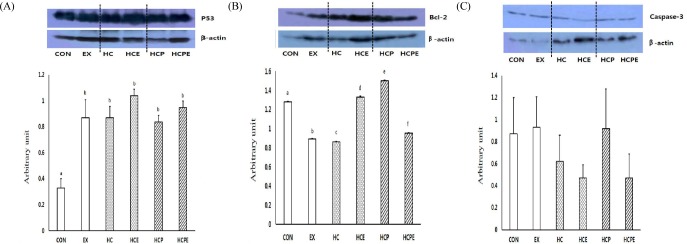
Difference of apoptosis protein expressions. A: P53 expression in gastrocnemius muscle, B: Bcl-2 expression in gastrocnemius muscle, C: Caspase-3 expression in gastrocnemius muscle. CON: normal diet control group; EX: normal diet and exercise training group; HC: high-cholesterol diet group; HCE: high-cholesterol diet and exercise training group; HCP: high-cholesterol and Pine Needle group; HCPE: high-cholesterol and pine needle diet and exercise training group; different letter means significance at p < 0.05, respectively.
DISCUSSION
Recently, several studies have reported on the antioxidant effect, anti-diabetic effect, and antiapoptotic effect of the combination treatment of exercise with functional foods [26,27]. In this study intended to investigate the effects of this treatment on antioxidant activities in muscle as well as cell death when consuming high-cholesterol diets, pine needle powder was added to the diet of rats and combined with 5 weeks of exercise.
ROS is generated in the environment surrounding cells and are also locally generated in muscular fibers. The continuous interaction between ROS generation and the cellular environment is the main factor that determines the state of equilibrium in cellular oxidation and reduction. It is known that a high cholesterol diet intake causes the increase of cell death, infection and oxidative stress by inducing mitogen-activated protein kinase (MAPK) activity [28,29]. In fact, research using hypercholesterolemia mice showed that the hydrolysis activities of MAPK and p38 is increased in hematopoietic stem cells, while oxidative stress is induced. Consequently, hindrance to differentiation due to aging and hematopoietic stem cell damage has an influence on the immune and inflammatory response [30]. Also, it has been reported that 30-40 minutes of aerobic exercise in healthy men increased the concentration of thiobarbituric acid reactive substances (TBARS) in thiobarbituric acid reactive substances plasma (TBARSpl), and thiobarbituric acid reactive substances erythrocytes (TBARSer) [31]. Other studies report that the formation of superoxide is increased in high intensity exercise. As a result, the overexpression of ICAM-1(Ig Cellular Adhesion Molecule-1) in the soleus muscle is induced, which reduces maintenance of aging, disease-exposure, muscle quantity, and muscle function [31-33]. In this study, analyzing the concentration of MDA, which is the index of lipid peroxidation in skeletal muscle, showed that during complex treatment with high cholesterol diets, pine needle powder intake and exercise, the HC group had significantly higher levels in comparison with other groups. On the contrary, the HCPE group demonstrated results that corresponded with previous studies by showing significantly lower values than other groups. These results suggest that the combination of pine needle powder intake and exercise may inhibit lipid peroxidation. The increase of MDA by high cholesterol diets is thought to be a result of the high content of saturated fatty acids increasing oxidative damage by promoting lipid peroxidation of bio membranes [35]. When rats consumed high cholesterol diets with added natural substances, antioxidant enzymes and antioxidant activity increased, whereas the peroxidation of lipid decreased. When fermented pine needle extract was added, oxidative damage in vivo was inhibited [36,37]. The above report also suggested that in in vivo experiments, the intake of pine needle influenced the concentration of Cu,Zn-SOD, and that glutathione played an important role [23]. In addition, it has been reported that administering the pine needle extract after inducing oxidative stress by tert-butyl hydroperoxide (t-BOOH) in HepG2 cells caused an increase in catalase (CAT) activity. When administering a high fat diet and pine needle extract for 5 weeks to ICR mice, their livers and kidneys showed lower lipid peroxide content due to the increase of CAT and glutathione activity [38]. It is known that the accumulation of lipid peroxides due to the damage of oxidative stress occurs during the lipid metabolism process, and causes all kinds of diseases by destroying cell membranes. However, it has been reported that the increased activity of GSH-Px, SOD and CAT, which are the antioxidant enzymes in the human body, play an important role in the removal of oxides by converting the oxygen anion generated by NADPH oxidase into H2O2 [39-42]. Due to previous studies showing that the strong antioxidant activities of such medicinal plants play an important role in the elimination and inhibition of free radicals, it was expected that in this study, the vitamin A, vitamin C and flavonoids contained in the pine needle would be instrumental in reducing the MDA content, and increasing the antioxidant activity [17,18,43]. It was concluded that at this time, continuous swimming exercise would have a synergy effect.
Apoptosis is cell death that is planned by the cells themselves. The death of these cells are influenced by various and complex phenomena in vivo [44]. Apoptosis is induced by the increase of oxidative stress due to external stimulus. Cell death can also be increased by the reduction of antioxidant capacity during heavy exercise [45,46]. In addition, it has been reported that a high cholesterol diet for 6 weeks induces apoptosis by increasing oxidative stress. High cholesterol diet intake for 3 months using C57BL/6 mice caused not only inflammatory activity and insulin resistance, but the increase of endoplasmic reticulum and apoptosis in endothelial blood vessel cells were also observed [47,48]. It is known that p53 selectively controls cell cycle block, DNA recovery, ageing and cell death, and reacts to endogenous stress [49]. Heavy exercises induce apoptosis by increasing the concentration of p53 which is the apoptosis marker of skeletal muscle [50,51]. Long-term exercise reduces the apoptosis in soleus muscles [52]. From this study, p53 which can be called the beginning marker for apoptosis, showed a significant difference between each group, with EX, HC and HCPE showing significantly lower results. Bcl-2, known as the antiapoptotic marker, showed significantly high value in all groups, with the exception of CON. HCE and HCPE showed especially high tendency for Bcl-2. Caspase-3, which is the final factor of apoptosis, did not show statistically significant difference, but the expression level in HCE and HCPE showed a tendency to decrease. Recent research results [53] show that the administration of pine needle extract in the cells that induce DNA damage reduce the expression of Bax protein by removing ROS in the cell, and inhibit the damage of cells by increasing the expression of the antiapoptotic factor Bcl-2. The report [54] also stated that the flavonoids contained in the pine needle extract inhibit the cell death factors by a medicinal function, the similarity will be able to be found. Thus, it is considered that the results of this study can be attributed to the influence of essential oil components, such as α-oinene, β-pinene and camphene which are the bioactive substances in pine needles, as well as the flavonoids and pinitol. This is in agreement with previous reports [56] suggesting that the chemical biological activities demonstrated by natural medicinal plants protects against apoptosis factors [55,56], and the antioxidation action of medicinal plants inhibit apoptosis [18,52].
CONCLUSION
The results of this study showed that the pine needle powder added diet as well as the low content of lipid peroxide increase the antioxidant defense capability of Mn-SOD, Cu,Zn-SOD. These are the elements inhibiting the activity of oxidation stress in the exercise group and may therefore effectively inhibit apoptosis. These results are attributed to the regular exercise and the action of the unique medicinal components contained in pine needles. In addition, it is considered that the availability of pine needles which can be more easily obtained from the surrounding environment will be increased due to the positive implications for the functionality of pine needle in this study. It is considered that pine needles will have beneficial effects as a functional food. We suggest that further research is needed on the bioactive substances contained in the pine needle.
Table 1.
The composition of experimental diet (g/kg diet)
| Constituents | Control diet | High-Cholesterol diet | + Pine Needle diet |
|---|---|---|---|
| Casein | 200 | 200 | 200 |
| Sucrose | 200 | 200 | 200 |
| Corn starch | 400 | 380 | 380 |
| Corn oil | 60 | 60 | 60 |
| Lard | 40 | 30 | 30 |
| Cholesterol | - | 30 | 10 |
| Cellulose | 50 | 50 | 50 |
| Mineral mix:AIN-76 | 35 | 30 | 30 |
| Vitamin mix:AIN-76 | 10 | 5 | 5 |
| D,L-methionine | 3.5 | 3.5 | 3.5 |
| Choline | 1.5 | 1.5 | 1.5 |
| Pine needle | - | - | 20 |
Mineral mix: AIN-76, Vitamin mix: AIN-76
REFERENCES
- 1.Ňygaard EB, Møller CL, Kievit P, Grove KL, Andersen B. Increased Fibroblast Growth Factor 21 expression in high-fat diet sensitive non-human primates (Macaca mulatta) Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.79. doi: 10.1038/ijo.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seo BY, Yoon YC, Paik HD, Park EJ. Effects of soymilk and whey protein fortified unripened cheese supplementation on lipid and antioxidant status in hypercholesterolemia SD rats. J. Basic Sciences. 2011;28:55–66. [Google Scholar]
- 3.Hatia S, Septembre-Malaterre A, Le Sage F, Badiou-Bénéteau A, Baret P, Payet B, Lefebvre d'Hellencourt C, Gonthier MP. Evaluation of antioxidant properties of major dietary polyphenols and their protective effect on 3T3-L1 preadipocytes and red blood cells exposed to oxidative stress. Free Radic Res. 2014 doi: 10.3109/10715762.2013.879985. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Nagel SA, Keuper M, Zagotta I, Enlund E, Ruperez AI, Debatin KM, Wabitsch M, Fischer-Posovszky P. Up-regulation of Bcl-2 during adipogenesis mediates apoptosis resistance in human adipocytes. Mol Cell Endocrinol. 2013;382:368–376. doi: 10.1016/j.mce.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Kwon HY, Kim JH, Lee WJ, Ju SB, Yoon SD. Utility Assessment of Whey Protein and β-carotene Supplementation for Muscle Mass, 1RM, Immunoglobulin and White Blood Cell Ratio during High Intensity Muscular Resistance Training. Journal of Sport and Leisure Studies. 2008;34:1217–25. [Google Scholar]
- 6.Kim JH, Kim YA. Effects of exercise intensity determined by grade of treadmill on apoptosis-related gens in rats. The Korea Journal of physical Education. 2007;46:487–496. [Google Scholar]
- 7.Pan MH, Ghal G, Ho CT. Review: Food bioactives, apoptosis, and cancer. Mol. Nutr. Food Res. 2008;52:43–52. doi: 10.1002/mnfr.200700380. [DOI] [PubMed] [Google Scholar]
- 8.Syu GD, Chen HI, Jen CJ. Severe exercise and exercise training exert opposite effects on human neutrophil apoptosis via altering the redox status. PLoS One. 2011;6: doi: 10.1371/journal.pone.0024385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanimura Y, Shimizu K, Tanabe K, Kono I, Ajisaka R. Effects of three consecutive days exercise on lymphocyte DNA damage in young men. Eur J Appl Physiol. 2010;110:307–314. doi: 10.1007/s00421-010-1499-2. [DOI] [PubMed] [Google Scholar]
- 10.Park JW, An SM, Lee MH, Kim KH, Kim IH, Huh K. Oxidative damage to DNA and modulation of antioxidant enzymes during ischemia/reperfusion insult to gerbil brain. Korea Biochem J. 1994;27:55–59. [Google Scholar]
- 11.Cho SH. Lipid Peroxidation and Antioxidant Nutrition. Korea Journal of Lipidology. 1993;3:23–32. [Google Scholar]
- 12.Choi IS, Cha EJ, Lee YR, Kim JK. Antioxidant and Anticancer Activities of Yak-Sun Tea Prepared by Oriental Medicinal Herbs. Korean J. Food & Nutr. 2012;25:447–453. [Google Scholar]
- 13.Song FL, Gan RY, Zhang Y, Xiao Q, Kuang L, Li HB. Total phenolic contents and antioxidant capacities of selected chinese medicinal plants. International Journal of Molecular Science. 2010;11:2362–3272. doi: 10.3390/ijms11062362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochwang'i DO, Kimwele CN, Oduma JA, Gathumbi PK, Mbaria JM, Kiama SG. Medicinal plants used in treatment and management of cancer in Kakamega County. Kenya J Ethnopharmacol. 2013;S0378-8741:00855–6. doi: 10.1016/j.jep.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Velusami CC, Agarwal A, Mookambeswaran V. Effect of Nelumbo nucifera Petal Extracts on Lipase, Adipogenesis, Adipolysis, and Central Receptors of Obesity. Evid Based Complement Alternat Med. 2013 doi: 10.1155/2013/145925. doi: 10.1155/2013/145925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montefusco-Pereira CV, de Carvalho MJ, de Araújo Boleti AP, Teixeira LS, Matos HR, Lima ES. Antioxidant, anti-inflammatory, and hypoglycemic effects of the leaf extract from Passiflora nitida Kunth. Appl Biochem Biotechnol. 2013;170:1367–1378. doi: 10.1007/s12010-013-0271-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Cui CB, Choi HT, Kim SH, Ham YA, Lee DS, Ham SS. Biological Activities of the Vaporized Liquid of Water-boiled Pine Needle. Korean J. Food Preserv. 2005;12:179–183. [Google Scholar]
- 18.Jeon JR, Kim JY, Lee KM, Cho DH. Anti-Obese Effects of Mixture Contained Pine needle, Black Tea and Green Tea Extracts. J. Korean Soc. Appl. Biol. Chem. 2005;48:375–381. [Google Scholar]
- 19.Cho YJ, Hou WN. Effects of dietary Bong-ip (Morus alba L.), Gam-chei(Glycyrrhizae glabra), Sol-ip(Pinus densiflora) and Dang-gi(Angelica gigas) on Serum Composition in Rats. KOREAN J. FOOD CULTURE. 2005;20:123–129. [Google Scholar]
- 20.Jeon JR, Kim JY. Effects of pine needle extract on differentiation of 3T3-L1 preadipocytes and obesity in high-fat diet fed rats. Biol Pharm Bull. 2006;29:2111–2115. doi: 10.1248/bpb.29.2111. [DOI] [PubMed] [Google Scholar]
- 21.Park YS, Jeon MH, Hwang HJ, Park MR, Lee SH, Kim SG, Kim M. Antioxidant activity and analysis of proanthocyanidins from pine (Pinus densiflora) needles. Nutr Res Pract. 2011;5:281–287. doi: 10.4162/nrp.2011.5.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryu HS, Kim HS. Studies on the Effects of Water Extract from Mixture of Pine Needles, Sedum sarmentosum Bunge, Hijkiaorme, Buckwheat and Perlla Leaves on the Immune Function Activation. Korean J. Food & Nutr. 2008;21:269–274. [Google Scholar]
- 23.Wingsle G, Karpinski S. Differential redox regulation by glutathione of glutathione reductase and CuZn-superoxide dismutase gene expression in Pinus sylvestris L. needles. Planta. 1996;198:151–157. doi: 10.1007/BF00197598. [DOI] [PubMed] [Google Scholar]
- 24.Kwak CS, Moon SC, Lee MS. Antioxidant, antimutagenic, and antitumor effects of pine needles (Pinus densiflora) Nutr Cancer. 2006;56:162–171. doi: 10.1207/s15327914nc5602_7. [DOI] [PubMed] [Google Scholar]
- 25.Yen GC, Duh PD, Huang DW, Hsu CL, Fu TY. Protective effect of pine (Pinus morrisonicola Hay.) needle on LDL oxidation and its anti-inflammatory action by modulation of iNOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. Food Chem Toxicol. 2007;46:175–185. doi: 10.1016/j.fct.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Park HS, Kim SK. The Effect of Aerobic Exercise Training and Taking Red Panax Ginseng on Antioxidant Enzymes and Lipid Superoxides in Diabetics. Journal of Sport and Leisure Studies. 2004;22:471–484. [Google Scholar]
- 27.Lee J, Kim WK, Cho HS. The Effects of 8weeks aerobic exercise on the expression of angiotensin ii and apoptosis in the kidney of l-name induced hypertensive rat. The Korean Journal of Physical Education. 2007;46:681–690. [Google Scholar]
- 28.Velusami CC, Agarwal A, Mookambeswaran V. Effect of Nelumbo nucifera Petal Extracts on Lipase, Adipogenesis, Adipolysis, and Central Receptors of Obesity. Evid Based Complement Alternat Med. 2013 doi: 10.1155/2013/145925. doi: 10.1155/2013/145925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mykkänen OT, Kalesnykas G, Adriaens M, Evelo CT, Törrönen R, Kaarniranta K. Bilberries potentially alleviate stress-related retinal gene expression induced by a high-fat diet in mice. Mol Vis. 2012;18:2338–51. [PMC free article] [PubMed] [Google Scholar]
- 30.Tie G, Messina KE, Yan J, Messina JA, Messina LM. Hypercholesterolemia induces oxidant stress that accelerates the ageing of hematopoietic stem cells. J Am Heart Assoc. 2014;3: doi: 10.1161/JAHA.113.000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sim MK, Wong YC, Xu XG, Loke WK. Des-aspartateangiotensin I attenuates ICAM-1 formation in hydrogen peroxide-treated L6 skeletal muscle cells and soleus muscle of mice subjected to eccentric exercise. Regul Pept. 2013;13:40–5. doi: 10.1016/j.regpep.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Sutkowy P, Woźniak A, Boraczyński T, Mila-Kierzenkowska C, Boraczyński A. The effect of a single Finnish sauna bath after aerobic exercise on the oxidative status in healthy men. Scand J Clin Lab Invest. 2013 doi: 10.3109/00365513.2013.860616. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal. 2011;15:247724–86. doi: 10.1089/ars.2011.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alipour M, Mohammadi M, Zarghami N, Ahmadiasl N. Influence of chronic exercise on red cell antioxidant defense, plasma malondialdehyde and total antioxidant capacity in hypercholesterolemic rabbits. J Sports Sci Med. 2006;5:682–91. [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MS, Chun SS, Choi JH. Effects of Turmeric (Curcuma longa L.) on Antioxidative Systems and Oxidative Damage in Rats Fed a High Fat and Cholesterol Diet. J Korean Soc Food Sci Nutr. 2013;42:570–6. [Google Scholar]
- 36.Lee CY, Mat Junit S, Abdulla MA, Abdul Aziz A. In vivo biochemical and gene expression analyses of the antioxidant activities and hypocholesterolaemic properties of Tamarindus indica fruit pulp extract. PLoS One. 2013;8: doi: 10.1371/journal.pone.0070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HJ, Kim SY, Park JH, Kim RY, Park EJ. Changes in the antioxidative and Antigenotoxic Effect after the Cooking Process of Sulgidduk Containing Pine needle Juice. KOREA J. FOOD COOKERY SCI. 2013;29:453–62. [Google Scholar]
- 38.Won SB, Jung GY, Kim J, Chung YS, Hong EK, Kwon YH. Protective effect of Pinus koraiensis needle water extract against oxidative stress in HepG2 cells and obese mice. J Med Food. 2013;16:569–76. doi: 10.1089/jmf.2012.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka Y, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. The Journal of Clinical Endocrinology & Metabolism. 2003;88:4673–6. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno T, Matsui H, Imamura A, Numaguchi Y, Sakai K, Murohara T, Okumura K. Insulin resistance increases circulating malondialdehyde-modified LDL and impairs endothelial function in healthy young men. International Journal of Cardiology. 2004;97:455–61. doi: 10.1016/j.ijcard.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 41.Kesavulu MM, Giri R, Kameswara Rao B, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes & Metabolism. 2000;26:387–92. [PubMed] [Google Scholar]
- 42.Lee H, Park WH, Cha YY. (2014). Effects of Cheunggihwadamhwan Extract on Lowering Lipid, Antioxidtion and Production of Proinflammatory Cytokines in Rats Fed on High Fat Diet. Journal of Korean Medicine Rehabilitation. 2014;24:1–13. [Google Scholar]
- 43.Hakimoğlu F, Kizil G, Kanay Z, Kizil M, Isi H. The effect of ethanol extract of Hypericum lysimachioides on lipid profile in hypercholesterolemic rabbits and its in vitro antioxidant activity. Atherosclerosis. 2007;192:113–22. doi: 10.1016/j.atherosclerosis.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Jeon SY, Seol DW. The role of mitochondria in apoptosis. BMB reports. 2008;41:11–22. doi: 10.5483/bmbrep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- 45.Kim SH, Kim JS, Yang KH. The Effect of Electroacupuncture and Acupuncture on the Change of Caspase-3 and Caspase-9 Immunoreactive Cell in Fixed Sprague Dawley Rat. Journal of Korea Sport Research. 2007;18:655–64. [Google Scholar]
- 46.Williams CA, Gordon ME, Betros CL, McKeever KH. Apoptosis and antioxidant status are influenced by age and exercise training in horses. Journal of Animal Science. 2008;86:576–83. doi: 10.2527/jas.2007-0585. [DOI] [PubMed] [Google Scholar]
- 47.Wang P, Xu TY, Guan YF, Zhao Y, Li ZY, Lan XH, Wang X, Yang PY, Kang ZH, Vanhoutte PM, Miao CY. Vascular smooth muscle cell apoptosis is an early trigger for hypothyroid atherosclerosis. Cardiovasc Res. 2014;102:448–59. doi: 10.1093/cvr/cvu056. [DOI] [PubMed] [Google Scholar]
- 48.Lu Y, Qian L, Zhang Q, Chen B, Gui L, Huang D, Chen G, Chen L. Palmitate induces apoptosis in mouse aortic endothelial cells and endothelial dysfunction in mice fed high-calorie and high-cholesterol diets. Life Sci. 2013;92:1165–73. doi: 10.1016/j.lfs.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Milisav I, Poljsak B, Suput D. Adaptive response, evidence of cross-resistance and its potential clinical use. Int J Mol Sci. 2012;13:10771–806. doi: 10.3390/ijms130910771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleem A, Adhihetty PJ, Hood DA. Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiol Genomics. 2008;37:58–66. doi: 10.1152/physiolgenomics.90346.2008. [DOI] [PubMed] [Google Scholar]
- 51.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: Effects of calorie restriction and life-long exercise. Experimental Gerontology. 2010;45:138–48. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong JB, Seo EW, Jeong HJ. Effect of extracts from pine needle against oxidative DNA damage and apoptosis induced by hydroxyl radical via antioxidant activity. Food Chem Toxicol. 2009;47:2135–41. doi: 10.1016/j.fct.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 53.Liu JJ, Sturrock RN, Benton R. Transcriptome analysis of Pinus monticola primary needles by RNA-seq provides novel insight into host resistance to Cronartium ribicola. BMC Genomics. 2013;16:884. doi: 10.1186/1471-2164-14-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reuland DJ, Khademi S, Castle CJ, Irwin DC, McCord JM, Miller BF, Hamilton KL. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic Biol Med. 2013;56:102–11. doi: 10.1016/j.freeradbiomed.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 55.Choi SJ, Kim YS, Song YJ, Kim JH, Kim JS. Screening of Korean Herbal Medicines with Inhibitory Activity on Advanced Glycation End Products Formation (XI) Kor. J Pharmacogn. 2013;44:372–8. [Google Scholar]
- 56.Kalekar SA, Munshi RP, Thatte UM. Do plants mediate their anti-diabetic effects through anti-oxidant and antiapoptotic actions? an in vitro assay of 3 Indian medicinal plants. BMC Complement Altern Med. 2013;5:257. doi: 10.1186/1472-6882-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]