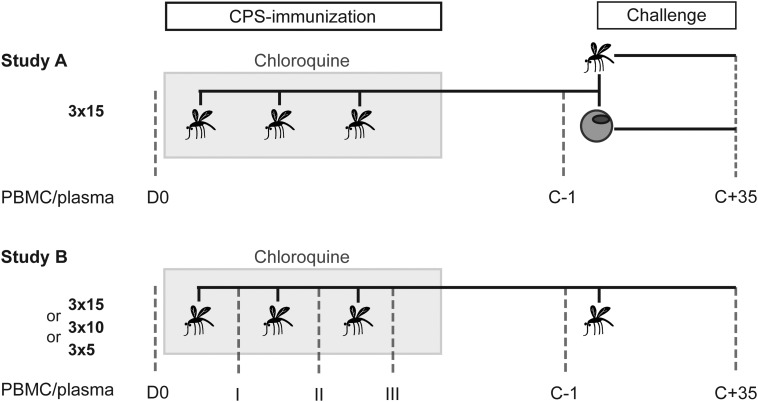
Figure 1.
Study design. Samples from 2 clinical chemoprophylaxis and sporozoites (CPS)–immunization trials were analyzed. In study A, volunteers were immunized on 3 occasions, separated by 4-week intervals, with bites of 15 Plasmodium falciparum–infected mosquitoes (3 × 15) while receiving a prophylactic regimen of the antimalarial drug chloroquine (gray box). For challenge infection 21 weeks after the last immunization (17 weeks after the final chloroquine dose), immunized volunteers were split into 2 groups, with one receiving P. falciparum–parasitized erythrocytes and the other exposed to bites from 5 infective mosquitoes. Plasma and peripheral blood mononuclear cell (PBMC) samples were obtained before immunization (D0), 1 day before challenge (C − 1), and 35 days after challenge (C + 35). In study B, 3 different immunization groups were exposed on 3 occasions, separated by 4-week intervals, to bites from 15 (3 × 15), 10 (3 × 10), or 5 (3 × 5) P. falciparum–infected mosquitoes while receiving chloroquine prophylaxis. All groups were challenged 19 weeks after the last immunization (15 weeks after the last chloroquine dose) with 5 P. falciparum–infected mosquito bites. Plasma and PBMC samples were collected before immunization (D0); 28 days after the first (I), second (II), and third (III) immunization; 1 day before challenge (C − 1), and 35 days after challenge (C + 35).