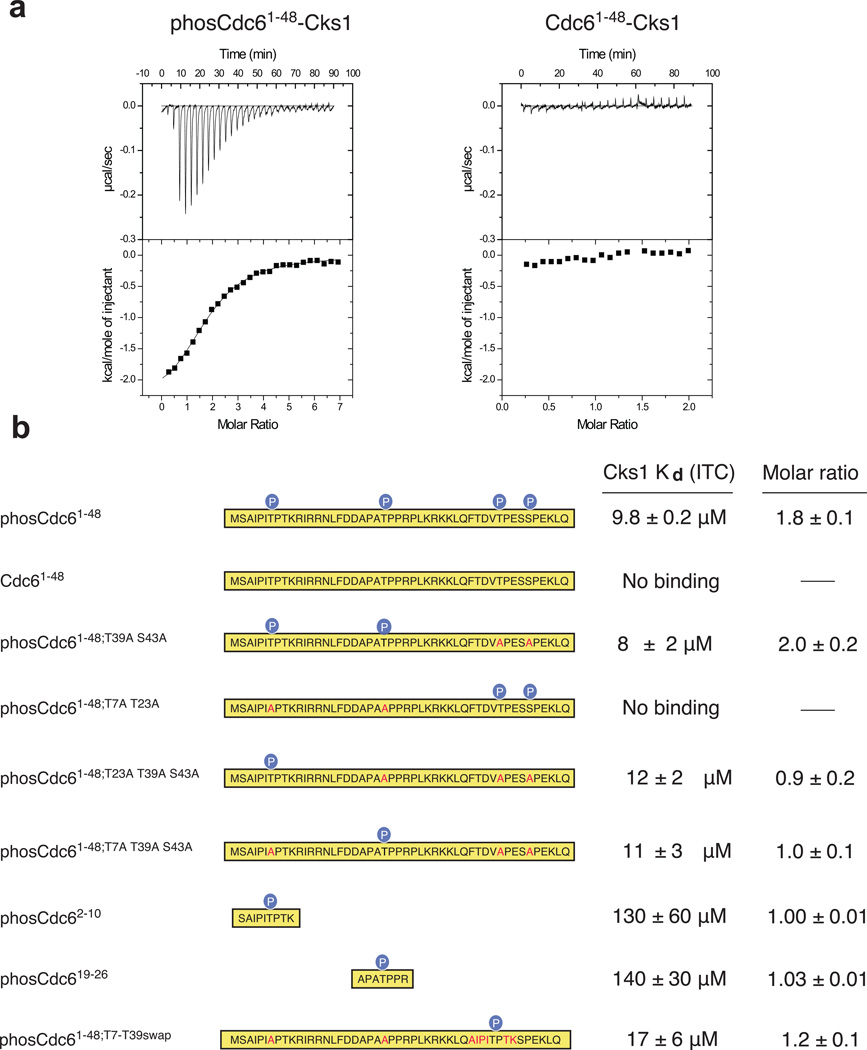
Figure 1.
Cks1 binds specific phosphorylated Cdk sites in the N-terminal domain of Cdc6. (a) Purified Cdc61–48 was phosphorylated with recombinant Cdk and affinity for Cks1 was measured by isothermal titration calorimetry. phosCdc61–48 binds Cks1 with Kd = 9.8 ± 0.2 µM (fit of data on left), while titration of unphosphorylated Cdc61–48 does not yield any binding heat (right). (b) ITC measurements with phosphorylated Cdc61–48 constructs and synthetic peptides demonstrate that two sites, T7 and T23, are each sufficient for Cks binding. Sequence mutations are highlighted in red within each construct. ITC data curves not shown in part (a) are shown in Supplementary Figure 1A–1G.