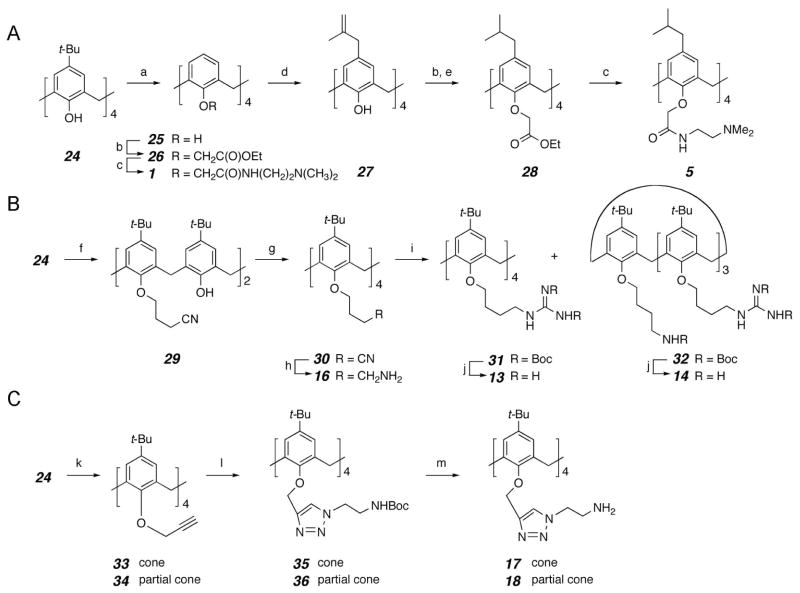
Scheme 1.
(A) synthesis of tertiary amine calixarene derivatives 1 and 5. (B) synthesis of guanidine calixarene derivatives 13 and 14 and primary amine calixarene derivative 16. (C) synthesis of triazole linked primary amine calixarene derivatives 17 and 18. Reaction conditions: a) AlCl3, PhOH, toluene, rt; b) ethyl bromoacetate, K2CO3, acetone, reflux; c) N,N-dimethylethylenediamine, toluene, reflux; d) i. NaH, 3-chloro-2-methylpropene, THF, DMF, 80 °C; ii. N,N-dimethylaniline, 200 °C; e) Pd/C, H2, 1atm, EtOAc, rt; f) 4-bromobutyronitrile, K2CO3, acetone, reflux; g) 4-bromobutyronitrile, NaH, DMF, 75 °C; h) NaBH4, CoCl2, MeOH; i) 1,3-bis(tert-butoxycarbonyl)-2-methyl-2-thiopseudourea, HgCl2, Et3N, CH2Cl2; j) TEA, 5% anisole in CH2Cl2, rt; k) NaH, propargyl bromide, THF, DMF, reflux; 1) N3(CH2)2NHBoc, ascorbic acid, NaOAc, CuSO4, t-BuOH, H2O, THF; m) TFA, 5% anisole in CH2Cl2, 0 °C to rt.