Abstract
BACKGROUND
Automated control of blood glucose (BG) has been a goal of type 1 diabetes (T1DM) therapy. The normal pancreas uses insulin and glucagon to regulate BG. Therefore, we developed a “closed-loop” BG control system utilizing both insulin and glucagon.
METHODS
Subcutaneous delivery of insulin lispro and glucagon was controlled by a computer algorithm that responded solely to BG levels every 5 min and incorporated a pharmacokinetic model for lispro. Eleven C-peptide negative subjects with T1DM were studied for 27 hours. Outcomes included mean BG, number and severity of hypoglycemic episodes, and comparison between measured and mode-estimated lispro levels.
RESULTS
In six subjects, the system achieved an aggregate 24-hour mean BG of 140 mg/dL, with no hypoglycemic events requiring treatment in 133 hours of control (nadir BG 66 mg/dL). In retrospect, their time-to-peak lispro levels were similar to that assumed by the algorithm (tmax 56-–72 min). Five subjects with generally slower lispro absorption (tmax 71–191 min) had at least one treatment-requiring hypoglycemic episode (17 episodes in 104 hours). Adjustment of the pharmacokinetic model parameters for lispro eliminated hypoglycemia in repeat experiments in the same five subjects, but with a mean BG of 173 mg/dL.
CONCLUSIONS
The original algorithm achieved near-normal mean BG with negligible hypoglycemia in subjects with rapid lispro kinetics. In subjects with delayed lispro absorption, modifying the pharmacokinetic assumptions prevented hypoglycemia, albeit with increased mean BG levels. These results demonstrate the feasibility of a bi-hormonal artificial endocrine pancreas. (ClinicalTrials.gov number, NCT00811317.)
Achieving and maintaining near-normal glycemia are critical in the long-term care of patients with diabetes mellitus. The Diabetes Control and Complications Trial and its long-term follow-up demonstrated the importance of intensive therapy in maintaining glycated hemoglobin (HbA1c) levels as close to the non-diabetic range as possible1-4. An HbA1c level less than 7%, which has been adopted internationally as a treatment goal5, reduces the development and progression of microvascular and cardiovascular complications by as much as 76%1, 4. Unfortunately, the intensive therapy that is required to achieve this goal is extremely demanding, necessitating frequent self-monitoring of blood glucose (BG) and multiple daily injections or use of an insulin pump. Even with physiologic insulin replacement in the form of basal-bolus therapy, substantial hyperglycemic excursions and hypoglycemia persist in most patients with type 1 diabetes (T1DM)6-8. Hypoglycemia could result in life-threatening consequences and limits the application of intensive therapy.
The development of a drug-delivery device that responds to glucose levels to automatically “clamp” BG levels in the non-diabetic range, frequently referred to as an “artificial endocrine pancreas”, has been a long-term goal. True “closed-loop” devices would require a stream of frequent BG measurements for operation. The prospect for development of such devices has been aided by recent improvements in minimally invasive continuous glucose monitoring (CGM) and by an improved understanding of the physiologic control of glycemia9-11. In individuals without diabetes mellitus, glucose levels are maintained between ~70–180 mg/dL through the interplay of insulin and glucagon secreted by the pancreatic islets12. Insulin is secreted in response to elevated glucose levels and other physiologic signals, and facilitates disposal of glucose into the liver and peripheral tissues. Glucagon counters the effects of insulin and increases glucose production by the liver, stabilizing glucose levels after meals and preventing hypoglycemia.
Based on these physiologic principles, we have developed a system for automated BG control by integrating a control algorithm with FDA-approved insulin pumps and an FDA-approved BG monitor (Fig. S1 of the Supplementary Appendix). This control system has previously been tested in a porcine model of insulin-deficient diabetes13, 14. We report here the first BG control results with this bi-hormonal artificial endocrine pancreas in human subjects with T1DM.
METHODS
SUBJECTS
The research protocol was approved by the Partners Human Research Committee and all participants gave written informed consent. Subjects were required to be 18 years of age or older and diagnosed with T1DM at least 5 years before enrollment. They had to have a HbA1c <8.5%, body mass index between 20 and 31 kg/m2, and be treated with insulin pump with a total daily insulin dose <1 unit/kg. Potential subjects were excluded if their C-peptide after a mixed-meal challenge was greater than 0.03 nmol/L1. The baseline characteristics of the subjects are shown in Table 1.
Table 1.
Baseline characteristics of subjects†.
| All subjects | Subjects requiring extra carbohydrates‡ | Subjects not requiring extra carbohydrates‡ | |
|---|---|---|---|
| Number | 11 | 5 | 6 |
| Sex | 7M / 4F | 5M | 2M / 4F |
| Age (years) | 40 ± 16 (19–71) | 47 ± 19 (19–71) | 34 ± 13 (20–50) |
| BM (kg) | 83 ± 13 (66–110) | 90 ± 12 (79–110) | 78 ± 11 (66–95) |
| BMI (kg/m2) | 28 ± 3 (22–31) | 27 ± 4 (22–31) | 28 ± 3 (22–31) |
| Diabetes Duration (years) | 23 ± 13 (6–46) | 30 ± 17 (6–46) | 17 ± 4 (9–21) |
| HbA1c (%) | 7.3 ± 0.8 (6.2–8.5) | 7.4 ± 1.0 (6.4–8.5) | 7.3 ± 0.7 (6.2–8.1) |
| Daily insulin dose (U/kg) | 0.6 ± 0.2 (0.3–1.0) | 0.5 ± 0.2 (0.3–0.8) | 0.7 ± 0.2 (0.5–1.0) |
| Stimulated c-peptide* (nmol/L) | < 0.03 | < 0.03 | < 0.03 |
Results are expressed as mean ± SD (range), unless otherwise stated.
Hypoglycemia was treated with extra carbohydrates (15 g) if BG remained below 60 mg/dL for a 20-minute period, below 50 mg/dL for a 10-minute period, or if subjects were symptomatic (see Supplementary Appendix).
All subjects had undetectable fasting and stimulated c-peptide, reported as less than the assay limit.
CLOSED-LOOP BG CONTROL SYSTEM
Briefly, the model-predictive control algorithm governed subcutaneous insulin lispro dosing with the goal of achieving a BG set-point of 100 mg/dL13, 14. The algorithm incorporated a pharmacokinetic model of the subcutaneous absorption and clearance of lispro. In the initial studies, the model parameters included a 33-minute time-to-peak (tmax) and 3.25-hour time to 95% clearance (t95%)13, 14. The lispro-dosing algorithm took into account both model-estimated plasma and subcutaneous lispro levels. A second set of experiments assumed tmax and t95% values of 65 min and 6.5 hours, respectively. A proportional-derivative control algorithm governed glucagon dosing with the goal of preventing or treating BG excursions below 100 mg/dL.
The control algorithm required only the subject’s weight for initialization and BG values every 5 min for online operation. Except for the change in PK parameters described above, the same algorithm parameters were used for all experiments. Insulin lispro (Humalog®, Eli Lilly, Indianapolis, IN) and glucagon (Eli Lilly) were delivered with insulin pumps with a minimum bolus size of 0.05 units (Deltec Cozmo®, Smiths Medical, St. Paul, MN) through subcutaneous infusion sets (Cleo® 90, Smiths Medical) inserted into the abdomen. Insulin lispro was delivered by two pumps, one containing U- 100 lispro and the second containing U-10 lispro (diluted with Sterile Diluent for Humalog®, Eli Lilly) to allow dosing resolution of 0.005 units. Glucagon, reconstituted according to the manufacturers instructions, was administered via a third pump with a dosing resolution of 0.5 μg. The maximum bolus size in any 5-minute interval was 2 units for lispro and 20 μg for glucagon. Plasma glucose levels were obtained in this proof-of-concept study with a device that automatically samples venous blood and assays it with glucose oxidase chemistry (GlucoScout™, International Biomedical, Austin, TX). The control algorithm was implemented in MATLAB® (The MathWorks™, Natick, MA) and run on a Powerbook computer (Apple, Inc., Cupertino, CA).
Closed-loop BG Control Experiments
Subjects were admitted to the Massachusetts General Hospital Clinical Research Center at 12:30 PM and continued to receive basal insulin from their own pump until 3:00 PM, when closed-loop control was begun. Intravenous catheters were inserted into each arm for blood sampling; one was connected to the GlucoScout™ and the other was used to obtain blood samples for later measurement of plasma lispro and glucagon levels, and for at least hourly BG measurements with the YSI 2300 Stat Plus™ Glucose Analyzer (YSI Life Sciences, Yellow Springs, OH). Hourly, paired GlucoScout™ and YSI values were required to be in agreement by ISO criteria15.
Subjects were fed three meals with specified size and macronutrient content to total 30 kcal/kg/day for men and 25 kcal/kg/day for women. Subjects completely consumed each meal in 30 min. The percentages of calories provided as carbohydrate were 45% at dinner, 60% at breakfast, and 50% at lunch. An 80-kg male would receive 122 grams at dinner and 90 grams of carbohydrate at breakfast and lunch. The meals were designed to provide a large carbohydrate challenge.
Hypoglycemia was defined as any plasma glucose level < 70 mg/dL. Oral carbohydrates (15 g) were given for treatment of hypoglycemia if the BG remained below 60 mg/dL for a 20-minute period, below 50 mg/dL for a 10-minute period, or if subjects were symptomatic (see Supplementary Appendix).
Laboratory Analyses
Blood for insulin and glucagon measurements was drawn into tubes containing EDTA and put immediately on ice. Plasma was isolated by centrifugation at 4°C and frozen within 30 min from the time of sampling. Insulin and glucagon were measured by immunoassay (Architect® insulin, Abbott Laboratories, Abbott Park, IL; Millipore, Billerica, MA). During screening, blood was obtained for HbA1c measurement by high performance liquid chromatography16 and serum was obtained for measurement of anti-insulin antibody titers by immunoassay17.
Pharmacokinetic Analyses
Plasma insulin and glucagon levels were initially measured in samples drawn at 10-minute intervals, but the interval for insulin was increased to 30 minutes and glucagon to 20 minutes since analyses demonstrated no substantive loss of information (data not shown). Models for PK behavior of lispro and glucagon were derived in each subject by fitting a summation of the exponential accumulation and decay functions for each bolus to the measured insulin lispro and glucagon levels using a least squares minimization protocol (see Supplementary Appendix). The tmax and t95% values were derived from the fitted function for each subject. These calculated values were not available to the control algorithm.
Statistical Analyses
The main outcomes were mean BG achieved, number of carbohydrate-treated hypoglycemic events, nadir BG during each experiment, percent of time in pre-specified BG ranges, and a comparison between measured and model-estimated lispro kinetics. Although the controller came online with fixed parameters that automatically adapted after initialization with the subject's weight13, 14, study results were calculated for the last 24 hours of each 27-hour experiment to reduce the influence of pre-experimental conditions on the outcome measures. The mean BG achieved over 24 hours was extrapolated to calculate the HbA1c expected if equivalent BG control were maintained over a 3-month period18. A hypoglycemic event started when the BG fell to < 70 mg/dL and ended when the BG returned to > 70 mg/dL. Statistical analyses were performed in Excel (Microsoft®, Redmond, WA).
RESULTS
Glycemic Control
Eleven subjects participated in at least one closed-loop experiment. Two patterns of BG control emerged in the 11 initial studies (See Figs. 1A and 1B for two examples and Figs. S2–S12 of the Supplementary Appendix for all profiles). There was no hypoglycemia requiring intervention in six subjects with relatively faster lispro PK and carbohydrate interventions were required in five subjects who had relatively slow lispro PK (Table 2).
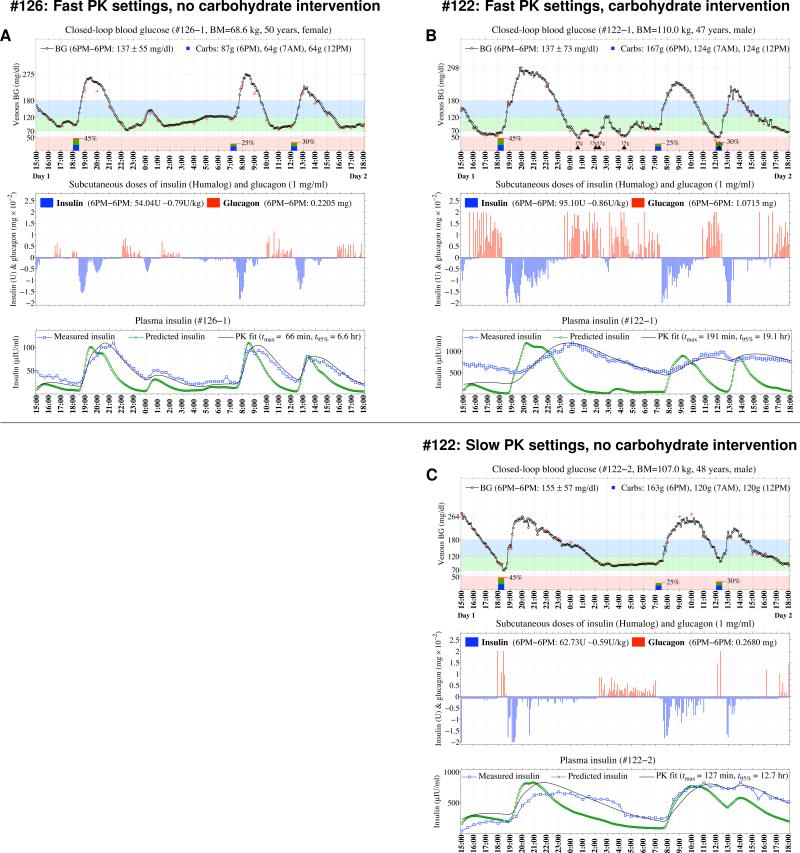
Figure 1. Representative Results from Three Closed-Loop Blood Glucose Control Experiments in Two Subjects.
Venous BG levels measured every 5 minutes with the GlucoScout™ (black circles) are shown in the top graph of Panel A for one of the six subjects who did not develop treatment-requiring hypoglycemia and, in retrospect, had relatively fast insulin lispo PK, and in the top graph of Panel B for one of the five subjects who required multiple 15-g carbohydrate treatments for hypoglycemia (indicated along the timeline by black triangles) and had slower lispro PK. Hourly confirmation values (red stars) obtained with the YSI are superimposed on the BG trace and meals are indicated along the timeline by rectangles with the percentage of daily calories of each meal indicated. Results from the repeat closed-loop experiment for the subject in Panel B using the slower PK parameter settings is shown in Panel C. The middle graph in each panel shows each bolus of insulin (vertical blue bars with negative amplitudes) and glucagon (vertical red bars with positive amplitudes) issued by the algorithm. The bottom graph in each panel shows the model-estimated (green circles) and measured (blue squares) insulin levels. The black line is the best fit of the bi-exponential PK model to the measured insulin levels (see Supplementary Appendix for further details).
Table 2.
Summary of closed-loop experiments.
| Subject ID# | BGavg±SD (mg/dl) | Projected HbA1c† (%) | [BGmin,BGmax] (mg/dl) | #Carb Interventions‡ | Inferred lispro PK | Percentage time (%) spent | Total lispro (U/kg) | Total glucagon (μg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tmax (min) | t95% (hr) | ≤70 | 70–120 | 70–180 | >180 | |||||||||
| CONTROLLER PK SETTING | FAST | 108* | 139 ± 60 | 6.5 | [73, 269] | — | 63 | 6.3 | 0 | 62 | 72 | 28 | 0.66 | 2.89 |
| 110 | 142 ± 50 | 6.6 | [67, 264] | — | 72 | 7.2 | 3 | 38 | 72 | 25 | 0.80 | 3.47 | ||
| 117 | 128 ± 52 | 6.1 | [66, 264] | — | 56 | 5.6 | 2 | 63 | 78 | 20 | 0.67 | 4.43 | ||
| 119* | 156 ± 57 | 7.1 | [80, 267] | — | — | — | 0 | 39 | 66 | 34 | 1.02 | 1.47 | ||
| 126 | 137 ± 55 | 6.4 | [74, 275] | — | 66 | 6.6 | 0 | 51 | 78 | 22 | 0.79 | 3.21 | ||
| 128 | 137 ± 41 | 6.4 | [74, 229] | — | 62 | 6.2 | 0 | 40 | 81 | 19 | 0.73 | 3.38 | ||
| Mean | 140 ± 9¶ | 6.5 | [72, 261] | — | 64 | 6.4 | 1 | 49 | 74 | 25 | 0.78 | 3.14 | ||
| 115 | 144 ± 65 | 6.7 | [47, 287] | 1 | 71 | 7.1 | 6 | 42 | 61 | 33 | 0.91 | 5.46 | ||
| 121* | — | — | [37, 357] | 3 | 111 | 11.1 | 14 | 18 | 28 | 58 | — | — | ||
| 122 | 137 ± 73 | 6.4 | [45, 298] | 5 | 191 | 19.1 | 19 | 35 | 52 | 29 | 0.86 | 9.74 | ||
| 129 | 164 ± 89 | 7.3 | [45, 360] | 2 | 132 | 13.2 | 9 | 37 | 53 | 38 | 1.17 | 6.71 | ||
| 132 | 129 ± 68 | 6.1 | [32, 269] | 6 | 78 | 7.8 | 18 | 40 | 59 | 23 | 0.84 | 10.3 | ||
| Mean | 144 ± 15¶ | 6.6 | [41, 314] | 3.4 | 97 | 9.7 | 13 | 34 | 51 | 36 | 0.95 | 8.05 | ||
| SLOW | 115 | 176 ± 57 | 7.7 | [99, 286] | — | 46 | 4.6 | 0 | 23 | 56 | 44 | 0.71 | 0.02 | |
| 121 | 179 ± 75 | 7.9 | [64, 319] | — | 80 | 8.0 | 3 | 26 | 50 | 47 | 0.77 | 2.75 | ||
| 122 | 155 ± 57 | 7.0 | [69, 264] | — | 127 | 12.7 | <1 | 38 | 65 | 35 | 0.59 | 2.51 | ||
| 129 | 198 ± 72 | 8.5 | [92, 333] | — | 141 | 14.1 | 0 | 19 | 46 | 54 | 0.85 | 0.32 | ||
| 132 | 157 ± 69 | 7.1 | [76, 293] | — | 50 | 5.0 | 0 | 46 | 65 | 35 | 0.68 | 2.67 | ||
| Mean | 173 ± 18¶ | 7.7 | [80, 299] | — | 89 | 8.9 | 1 | 30 | 56 | 43 | 0.72 | 1.65 | ||
Statistics are reported for 24 hours, starting at 6 P.M. on admission day and ending at 6 P.M. on the next day, except in (three) cases where the experiment was discontinued earlier.
Reported HbA1c values are projections based on mean BG17.
Carbohydrate interventions were administered according to protocol.
Experiments were discontinued due to loss of IV access in #108 (statistics are reported for 15.75 hours) and #119 (statistics are reported for 21.33 hours), and due to intervention with IV dextrose in the first experiment in #121 (statistics are reported for 7.5 hours).
Mean and standard deviation in BG across subjects were computed based on the individual mean BG values.
For the six subjects, the closed-loop system achieved an aggregate mean BG of 140 mg/dL with only two episodes of asymptomatic biochemical hypoglycemia in 133 hours of closed-loop control. The nadir BG for these experiments was 66 mg/dL. Seventy-four percent of study time was spent with BG in the American Diabetes Association (ADA) glycemic target range of 70–180 mg/dL5, and less than 1% of time was spent below 70 mg/dL (Table 2). There was an anticipated post-prandial hyperglycemic excursion after each meal since the algorithm is entirely reactive and issues insulin only after the glucose level begins to rise, and there is a delay in the absorption of subcutaneous insulin.
Most of the prandial insulin was provided in the hour following initiation of the meal (Fig. 1). The control algorithm issued additional insulin if the BG remained out of range and the insulin in the subcutaneous depot was estimated by the model to be insufficient to regulate the BG excursion. The total amount of insulin provided over the 24 hours averaged 0.78 units/kg in these six subjects. If the slope of the fall in BG was steep as it approached 100 mg/dL, or if BG fell below 100 mg/dL, the controller issued glucagon, which was typically followed by a rapid change in the slope of the BG (Fig. 1A, 1C). Glucagon doses were small relative to the typical 1 mg dose used clinically to treat hypoglycemia; the largest single dose in a 5-min interval was 20 μg. The total glucagon administered averaged 3.14 μg/kg/24 hours over all experiments in this group (total dosage 120–377 μg/24 hours, Table 2).
The performance of the closed-loop system and resultant glucose pattern in the initial studies of the other five subjects was markedly different (Fig. 1B and Figs. S8–S12 of the Supplementary Appendix). Each of these subjects developed hypoglycemia (20 events of BG < 70 mg/dL in 104 hours of closed-loop control), including at least one episode requiring oral carbohydrate treatment (mean 3.4 doses of 15 g carbohydrates per experiment, total of 17 carbohydrate interventions, Table 2). One experiment was terminated early due to the need for 3 doses of oral carbohydrate in 1 hour and intravenous dextrose administration. The aggregate mean level of glycemia was not significantly different between this group and the other six subjects without hypoglycemia (144 vs 140 mg/dL), owing to more time spent in the hypoglycemic range (< 70 mg/dL, 13% vs less than 1%, p = 0.007) and in the hyperglycemic range (> 180 mg/dL, 36% vs. 25%, p = 0.12). The hypoglycemic events were typically in the late postprandial period, despite more glucagon delivery in this group than in the other six subjects (mean 8.05 μg/kg/24 hours vs. 3.14 μg/kg/24 hours, p = 0.02). The attenuation or reversal in the downward BG trend after glucagon administration noted in the other group was not evident in these subjects.
Insulin Pharmacokinetics
Analysis of insulin pharmacokinetics (PK) in each of the closed-loop experiments suggested that differences in the rate of lispro absorption and clearance were responsible for inter-subject variability in closed-loop system performance. There was a large variation in lispro PK between subjects, with tmax ranging from 5.6 to 191 min and t95% ranging from 5.6 to 19.1 hours (Table 2). The subjects without significant hypoglycemia exhibited tmax values ranging from 56 to 72 min. The five subjects with hypoglycemia requiring carbohydrate treatment had tmax values ranging between 71 and 191 min. The subject in this group with the lowest tmax (71 min) required carbohydrate treatment only once, while all of the other subjects in this group with longer tmax values required carbohydrate treatment repeatedly.
Figures 1 and 2 compare the measured lispro concentration profiles with the online model-estimation of these profiles that was used by the control algorithm in calculating lispro dosing. There was generally good agreement between the model-estimated and measured insulin levels, especially with regard to the rise in insulin levels in the immediate post-prandial period for the six subjects who did not develop hypoglycemia requiring treatment (Fig. 1A and Figs. S2–S7 of the Supplementary Appendix). Conversely, in the five subjects who did require carbohydrate intervention, there was a substantial disparity between the model-estimated and measured insulin levels. In particular, the observed peak in plasma lispro concentration was markedly delayed compared to the controller estimation (Fig. 1B and Figs. S8–S12 of the Supplementary Appendix). The control algorithm continued to issue more insulin doses in response to the resulting continued hyperglycemia. Moreover, the algorithm, configured with the fast PK settings, could not anticipate the subsequent absorption of insulin that had accumulated in the subcutaneous depot. Consequently, the plasma insulin concentration in the late post-prandial period was excessive and resulted in hypoglycemia, apparently refractive to glucagon. Summary BG and plasma-insulin profiles for all of the initial studies are shown in Fig. 2A–2D.
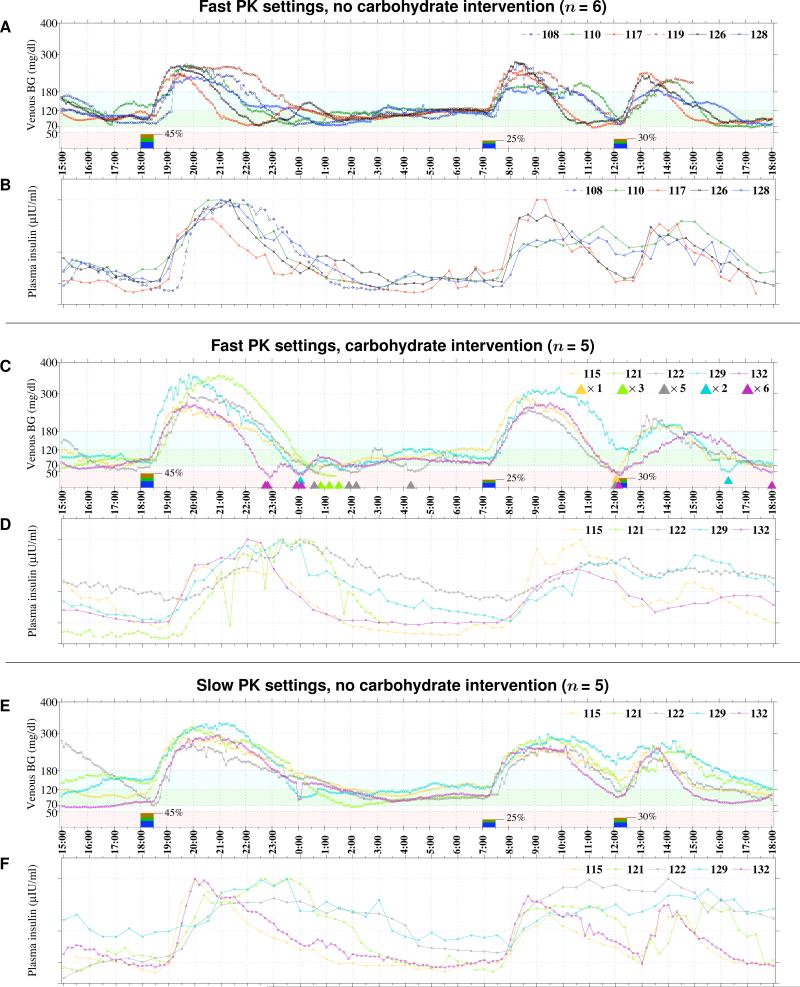
Figure 2. Blood Glucose and Plasma Insulin Levels from Closed-Loop BG Control Experiments in All 11 Subjects.
Venous BG and measured insulin lispro levels are shown, respectively, in Panels A and B for the six subjects who did not develop treatment-requiring hypoglycemia, and in Panels C and D for the five subjects who required one or more carbohydrate treatments for hypoglycemia during the initial experiments using the controller configured with the fast lispro PK parameter settings (tmax=33 min), and in Panels E and F for the repeat experiments using the controlle configured with the slow PK setting (tmax=65 min). Each 15-g carbohydrate intervention for hypoglycemia is indicated along the timeline in Panel C with a color-coded triangle.
Repeat Closed-loop Studies Using a Modified Pharmacokinetic Model for Lispro
To test the hypothesis that disparity between the model-estimated and measured insulin levels was responsible for hypoglycemia, we adjusted the PK parameter settings to a lispro tmax of 65 min, twice the value used in the initial studies, and performed repeat closed-loop experiments in each of the five subjects who had required carbohydrate treatment for hypoglycemia (Figs. 2E and 2F, and Figs. S13–S17 of the Supplementary Appendix). In each repeat experiment, closed-loop BG control was achieved without any hypoglycemia requiring carbohydrate intervention, albeit at a cost of slightly higher mean BG levels (Table 2). Plasma insulin levels estimated on-line by the controller corresponded more closely to measured insulin levels than in the initial studies, and less glucagon was required (1.65 vs. 8.05 mg/kg, p = 0.006)(Table 2).
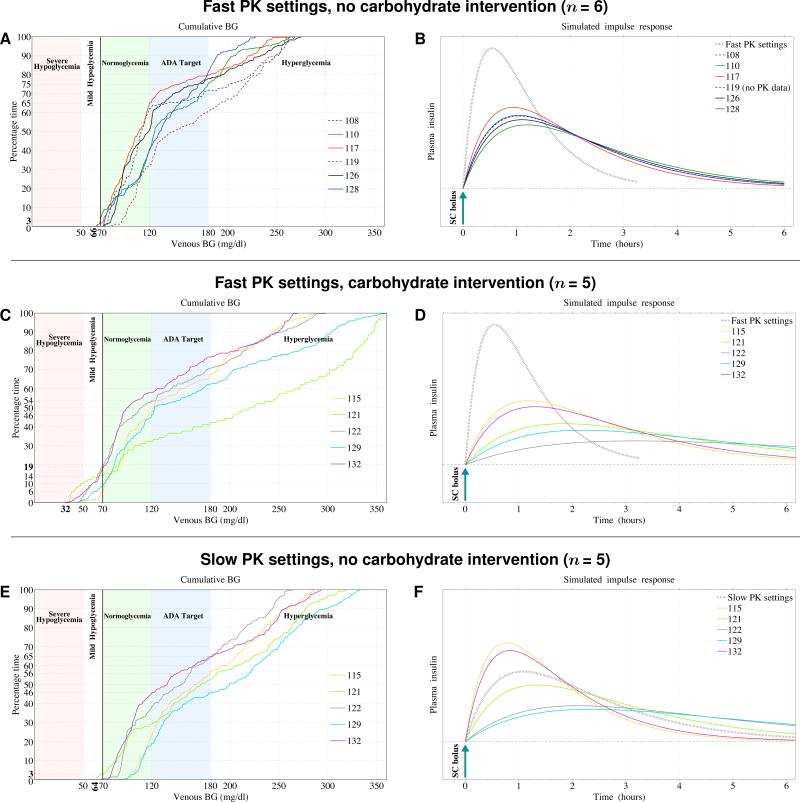
The cumulative BG traces shown in Fig. 3A and 3C demonstrate that the faster PK group spent the majority of time on average (74%) in the ADA glycemic target range with no treatment-requiring hypoglycemia, whereas in the slower PK group, there was both more hypo- and hyperglycemia and only 51% of time was spent in the ADA target range. In the repeat experiments with slow PK settings (Fig. 3E), the distribution of BG results was compressed, with no hypoglycemia. Figures 3B, 3D, and 3F show a comparison between the algorithm's PK settings and graphical representations of the lispro PK for each subject derived from their measured insulin levels. It is evident that when there is less disparity between the model-estimated PK and the subject's PK (compare Figs. 3B and 3D), glycemic control is improved without treatment-requiring hypoglycemia (compare Figs. 3A and 3C). In the repeat experiments, model-estimated PK was in closer agreement with each subject's PK (Fig. 3F), and treatment-requiring hypoglycemia was eliminated (Fig. 3E).
Figure 3. Cumulative BG Levels and Lispro PK from Closed-Loop BG Control Experiments in All 11 Subjects.
Cumulative venous BG levels are shown in Panel A for the six subjects who did not develop treatment-requiring hypoglycemia, and in Panel C for the five subjects who required one or more carbohydrate treatments for hypoglycemia during the initial experiments using the controller configured with the fast lispro PK parameter settings (tmax=33 min), and in Panel E for the repeat experiments using the controller configured with the slow PK settings (tmax=65 min). Shown in parallel with Panels A, C, and E are the corresponding graphical representations of each subjects’ simulated insulin profiles (Panels B, D, and E) derived, retrospectively, from the measured lispro levels in Panels A, C, and E and portrayed as the lispro levels after a single insulin bolus (see Supplementary Appendix for further details). Whereas the fast PK parameter settings resulted in model-estimated PK that was faster than any subject's measured PK (Panels B and D), the slow PK parameter settings resulted in model-estimated PK that fell between the fastest and slowest subjects’ measured PK in the repeat experiments (Panel F). When the measured insulin PK (solid curves in Panels B and F) was closer to the model-estimated PK used by the control algorithm (black hatched curve in Panels B and F), the time spent in the ADA glycemic target range was greater, with no treatment-requiring hypoglycemia (Panels A and E); however, when the measured insulin levels were discrepant from the model-estimated PK used by the control algorithm (Panel D), blood glucose levels were both higher and lower than the ADA target range with hypoglycemia requiring intervention (Panel C).
DISCUSSION
We have demonstrated the feasibility of a bi-hormonal closed-loop BG control system. Near-normal mean BG levels were achieved without feed-forward information or pre-treatment for the very high-carbohydrate meals, and without hypoglycemia in the subjects with faster insulin PK. In subjects with slower insulin absorption, adjustment of the algorithm's PK parameters prevented hypoglycemia at the cost of modestly higher average BG values. Other clinical trials testing closed-loop control using subcutaneous insulin infusion have reported multiple episodes of hypoglycemia in several subjects, but did not attempt any repeat experiments19, 20. These studies neither addressed the cause of the hypoglycemia, nor provided a potential remedy to mitigate it. In contrast, we have identified discordance between measured and model-estimated insulin levels as a possible cause of the hypoglycemic episodes that we observed and were able to eliminate hypoglycemia in the same subjects by modification of the PK parameters.
The BG values were generally in the non-diabetic range between meals and overnight, but higher than normal after meals. The post-prandial glucose excursions are a function of the glucose control algorithm being entirely reactive (i.e. delivering insulin only in response to a glucose rise) and the relatively slow absorption of subcutaneous insulin. The system, therefore, requires some time to “catch-up” after a carbohydrate load. The inclusion of glucagon in our system was designed to imitate normal physiology and prevent the post-prandial hypoglycemia that has been seen in closed-loop studies using insulin only19, 20. However, when the disparity between measured and model-estimated lispro levels was too large, and insulin accumulated, the small doses of glucagon delivered by the controller were not sufficient to prevent hypoglycemia. In these same subjects, glucagon was apparently effective in preventing hypoglycemia after adjustment of the PK parameters.
There are several potential ways to address the post-prandial hyperglycemia that we will test in future studies. For example, providing a small pre-meal insulin bolus would increase insulin levels in a more timely fashion than the reactive algorithm alone. Additionally, the glucagon control algorithm could be modified to provide escalating doses of glucagon if the BG response was not adequate.
Insulin analogs with more rapid PK and less variability are desirable as they would potentially lower glucose excursions without causing hypoglycemia. In the absence of faster analogs, we have tested the slower PK parameters in two of our subjects with faster PK. The closed-loop experiments (Figs. S18 and S19 of the Supplementary Appendix) showed no hypoglycemia and only a minimal increase in mean BG (~11 mg/dL), suggesting that these PK parameters could be widely applicable.
We performed these proof-of-principle studies with devices that are FDA-approved with the hope that if the algorithm were effective, subsequent development of a practical artificial endocrine pancreas would be facilitated. While the use of a venous BG input signal is not practical for outpatient use, it allowed us to assess the performance of our control algorithm independent of confounding owing to a less accurate glucose input signal. However, in parallel we measured interstitial-fluid glucose levels with the three commercially available CGM devices to examine the differences between the laboratory-quality plasma glucose measurements and the interstitial values obtained with CGM. At least one device showed sufficiently high accuracy and reliability to warrant testing as the sensing limb to the control system (data not shown) and future studies will use CGM data as the sole input signal. In addition, our subjects were studied in a controlled environment in which they were sedentary and ate standardized meals, albeit with a high carbohydrate content. The performance of the control system during free activity and aerobic exercise will also be explored in future studies. The current results suggest that closed-loop control of BG levels to the near-normal range without the need for frequent monitoring and injections, and without risk of hypoglycemia, will be feasible with a bi-hormonal artificial endocrine pancreas.
Supplementary Material
Acknowledgments
Supported by grants from the Juvenile Diabetes Research Foundation (22-2007-1801, to Dr. Damiano), the Wallace H. Coulter Foundation (sub-award from BU–Coulter Translational Partnership Award to Drs. Damiano and Russell), the Charlton Fund for Innovative Research in Diabetes (to Dr. Nathan) and the NIH National Center for Research Resources through the GCRC and CTSC programs (RR01066 and 1-UL1-RR025758-01).
We thank the volunteers for their time and enthusiasm, the nurses and laboratory staff of the MGH Clinical Research Center for their dedicated effort and careful execution of the experimental protocol, Mary Larkin, Richard Pompei, Ellen Nicholson, Gail Winning, Nancy Kingori, and John Jiang for organizational and logistical support, and Joseph Moy and Patrick Sluss of the MGH Reproductive Endocrine Laboratory for performing insulin and glucagon assays.
Footnotes
No potential conflicts of interest relevant to this article were reported other than the following: Drs. El-Khatib and Damiano have pending patents on the closed-loop algorithm and Drs. Russell, Damiano, El-Khatib, and Nathan have a patent pending on predicting insulin pharmacokinetics based on anti-insulin antibody titers.
REFERENCES
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342(6):381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. Jama. 2002;287(19):2563–9. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46(2):271–86. [PubMed] [Google Scholar]
- 7.Leese GP, Wang J, Broomhall J, et al. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26(4):1176–80. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 8.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic Control in Youth with Diabetes: The SEARCH for Diabetes in Youth Study. J Pediatr. 2009 doi: 10.1016/j.jpeds.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinman B. The physiologic replacement of insulin. An elusive goal. N Engl J Med. 1989;321(6):363–70. doi: 10.1056/NEJM198908103210605. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther. 2008;10(4):232–44. doi: 10.1089/dia.2008.0016. [DOI] [PubMed] [Google Scholar]
- 11.Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 12.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48(5):1198–214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 13.El-Khatib FH, Jiang J, Damiano ER. A feasibility study of bihormonal closedloop blood-glucose control using dual subcutaneous infusion of insulin and glucagon in ambulatory swine. J Diab Sci Technol. 2009;3(4):789–803. doi: 10.1177/193229680900300428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Khatib FH, Jiang J, Damiano ER. Adaptive closed-loop control provides bloodglucose regulation using dual subcutaneous insulin and glucagon infusion in diabetic swine. Diabetes Science and Technology. 2007;2:181–92. doi: 10.1177/193229680700100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ISO 15197 [8/30/2009];International Organization for Standardization. 2003 2003 (2003, at http://www.iso.org/iso/catalogue_detail.htm?csnumber=26309.)
- 16.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310(6):341–6. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 17.Vardi P, Dib SA, Tuttleman M, et al. Competitive insulin autoantibody assay. Prospective evaluation of subjects at high risk for development of type I diabetes mellitus. Diabetes. 1987;36(11):1286–91. doi: 10.2337/diab.36.11.1286. [DOI] [PubMed] [Google Scholar]
- 18.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–8. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–50. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 20.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–9. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.