Abstract
• Background and aims Pollination is a critical stage in plant reproduction and thus in the maintenance and evolution of species and communities. The Caatinga is the fourth largest ecosystem in Brazil, but despite its great extent and its importance few studies providing ecological information are available, with a notable lack of work focusing on pollination biology. Here, general data are presented regarding the frequency of pollination systems within Caatinga communities, with the aim of characterizing patterns related to floral attributes in order to make possible comparisons with data for plant communities in other tropical areas, and to test ideas about the utility of syndromes. This paper also intends to provide a reference point for further studies on pollination ecology in this threatened ecosystem.
• Methods The floral traits and the pollination systems of 147 species were analysed in three areas of Caatinga vegetation in northeastern Brazil, and compared with world-wide studies focusing on the same subject. For each species, floral attributes were recorded as form, size, colour, rewards and pollination units. The species were grouped into 12 guilds according to the main pollinator vector. Analyses of the frequencies of the floral traits and pollination systems were undertaken.
• Key Results Nectar and pollen were the most common floral resources and insect pollination was the most frequent, occurring in 69·9 % of the studied species. Of the entomophilous species, 61·7 % were considered to be melittophilous (43·1 % of the total). Vertebrate pollination occurred in 28·1 % of the species (ornithophily in 15·0 % and chiropterophily in 13·1 %), and anemophily was recorded in only 2·0 %.
• Conclusions The results indicated that the pollination systems in Caatinga, despite climatic restrictions, are diversified, with a low percentage of generalist flowers, and similar to other tropical dry and wet forest communities, including those with high rainfall levels.
Key words: Floral traits, floral rewards, pollination syndromes, dry forests, Caatinga, northeast Brazil
INTRODUCTION
Morphological, physiological and ecological floral traits are related to pollination vectors, and an analysis of these attributes can help in the prediction of the pollinator of a species (cf. Faegri and Pijl, 1979; Waser, 1983; Dafni and O'Toole, 1994; Endress, 1994; Proctor et al., 1996). The relationships observed characterize pollination syndromes (sensu Vogel, 1954; Faegri and Pijl, 1979), and provide a valuable guide to studies on reproductive ecology. However, while some degree of variation is acceptable, it should be noted that floral traits are not infallible indicators of species' pollinators. In spite of the limits of studies based on floral syndromes, for example as mentioned by Herrera (1995) and Ollerton (1996), they contribute to the understanding of pollination biology at a community level (Rebelo et al., 1985; Ramirez et al., 1990; Dafni and O'Toole, 1994; Tabla and Bullock, 2002; Muchhala and Jarrín-V, 2002), to comparisons of pollination system frequency among floras and vegetation types, and also towards directing more specific research. According to Cruden (1997) and Johnson and Steiner (2000), floral syndromes can be effectively tested by broad-scale comparisons between multiple floral traits and pollinators.
Floral morphology can exclude some floral visitors and attract potential pollinators, since the diversity of floral types is also associated with the sensorial development of pollinators, particularly those related to the capacity to distinguish between and to memorize certain visual and olfactory patterns (Leppik, 1968; Faegri a0nd Pijl, 1979; Ramirez et al., 1990; Chittka et al., 2001; Gegear and Laverty, 2001). In addition, there are some interdependent relationships between flower and pollinator sizes (Feinsinger and Colwell, 1978; Frankie et al., 1983). The occurrence of different floral resources indicates the presence of different kinds of pollinators, sometimes quite specialized as, for example, certain Anthophoridae and the Euglossini bees that pollinate flowers that offer oils, perfumes or resins as a reward (Vogel, 1966, 1974; Dressler 1968a, b; Armbruster, 1984).
Pollination is a critical stage in plant reproduction and thus in the maintenance and evolution of species and communities. Although most studies of pollination focus on one or a few species, a community perspective is important for pointing the way for specific studies, for comparing different ecosystems, for understanding sharing and competition for resources and their effects on community structure, and for guiding conservation programmes in threatened and fragmented ecosystems (e.g. Saunders et al., 1991; Rathcke and Jules, 1993; Aizen and Feinsinger, 1994; Murcia, 1995, 2002; Kearns and Inouye, 1997; Ranta et al., 1998; Renner, 1998).
The Caatinga is an ecological region of northeastern Brazil occupied by tropical dry forest and scrub vegetation, pastures and agricultural systems (Sampaio, 1995; MMA, 2002). It is an equatorial region of extreme drought, few wet months per year (3–5 months) with low annual rainfall levels (e.g. 500–750 mm year−1), and extreme supra-annual variation in rainfall. Annual temperature averages 23–27 °C and the region suffers water deficit during most of the year (for details see Sampaio, 1995; Rodal and Melo, 1999).
The Caatinga is the fourth largest ecosystem in Brazil, after the Amazonian rain forest, the Cerrado, and the Atlantic forest (Aguiar et al., 2002), covering 734 478 km2 (MMA, 2002), which is almost 50 % of the northeastern region and 8·6 % of the country (Hueck, 1972). Recently the Caatinga was recognized as an ‘Earth's last wild place’, and included as one of the 37 ‘Wilderness Areas of the World’ (Gil, 2002).
Despite its great extent and considering the importance of the Caatinga region for Brazil, few studies with ecological information are available, with a notable lack of publications focusing on the biology and the dynamics of its species (see Leal et al., 2003 for review). Studies of pollination processes of Caatinga species are scarce (see Machado, 1996 and Machado and Lopes, 2002 for review), and these investigations focus mainly on individual species (Pinheiro et al., 1991; Vogel and Machado, 1991; Machado and Sazima, 1995; Lewis and Gibbs, 1999; Locatelli and Machado, 1999; Kiill and Ranga, 2000; Piedade-Kiill and Ranga, 2000; Kiill and Drummond, 2001; Quirino and Machado, 2001; Machado et al., 2002). Studies on pollination ecology of the Caatinga at a community level are even rarer (see Machado 1990, 1996; Machado and Lopes, 2002).
The Caatinga areas are, year by year, suffering increasing anthropic destruction, resulting in the loss of native fauna and flora (Sampaio, 1995; MMA, 2002). As a consequence, the plant species composition and vegetation structure are being significantly altered, with the habitat being reduced to small fragments. There are few previous biological and ecological studies relating to this. A broad knowledge of plant reproductive biology may be essential for the maintenance of the biodiversity of fragmented areas (Bawa, 1990) and for management projects in this ecosystem.
This work presents general data on the occurrence and the frequency of pollination systems in Caatinga communities. We aimed to characterize patterns related to the floral attributes of the taxa of this type of vegetation, to make possible comparisons with data for plant communities in other tropical areas, and to test ideas about the utility of syndromes and trends in specificity. This paper also intends to provide a reference point for further studies on pollination ecology in this threatened ecosystem.
MATERIALS AND METHODS
Study sites and species
The study was carried out on species referred in the literature as occurring in Caatinga vegetation (Andrade-Lima, 1989; Ferraz et al., 1998; Rodal and Melo, 1999), and most of them were observed in three municipalities (Alagoinha, Buíque and Serra Talhada) located in the rural zone of Pernambuco State (PE), northeastern Brazil (Fig. 1). These areas have different physiognomies, and published floristic and phytosociological surveys are available (Ferraz et al., 1998; Rodal et al., 1998; Figueiredo et al., 2000).
Fig. 1.
Location of the studied sites (arrows) in Pernambuco state (detail), Northeast Region (light grey) of Brazil (dark grey).
One of the areas, Sítio Riacho, is situated in the municipality of Alagoinha-PE (8°27′S, 36°46′W; 762 m a.s.l.), approx. 200 km from the coast, and comprises about 80 ha. The climate of this site is semiarid, of the Bs s′h′ type according to the classification of Köppen. The number of dry months varies from 5–7, with mean annual temperature and precipitation approx. 22 °C and 550 mm, respectively (Griz and Machado, 2001). The vegetation is characterized by a shrubby, dense Caatinga (Egler, 1951), with a predominance of amply ramified and thorny shrubs.
The second area is the Vale do Catimbau (8°67′S, 37°11′W), a Brazilian National Park located in the municipality of Buíque-PE, and 285 km from the coast. The altitude in the area ranges between 800–1000 m a.s.l., and the mean annual precipitation is 1095·9 mm, with rain from January to June but mostly from April to June; the mean annual temperature is 25 °C (SUDENE, 1990). The vegetation of this valley is very unusual, with many plant species not found in other Caatinga areas, some of them normally found in other types of ‘open’ vegetation such as ‘Campos Rupestres’ (Rodal et al., 1998).
The most inland area (approx. 700 km from the coast) is Fazenda Saco located in the municipality of Serra Talhada-PE (7°59′S, 38°19′W), and is an experimental station of the Agricultural Research Company of Pernambuco (IPA). The altitude in the area is approx. 600 m a.s.l. and the mean annual precipitation is 650 mm, with high variation from year to year. Generally, a pronounced dry period occurs from June to December, with rains from January to May. The mean annual temperature is 26 °C, with little monthly variation (Machado et al., 1997a). The vegetation is dominated by shrubs, the majority around 3–4 m tall with stem diameter at breast height (DBH) of 3–6 cm, with a few trees reaching 15 m high and DBH of 60 cm (Ferraz et al., 1998). As in the first area, the herbaceous stratum is not very dense, and is composed mainly of annual plants that grow only in the rainy period.
In addition to observations made on field trips, during which direct observations of floral biology and pollinators were made together with collections, some analyses were conducted using specimens deposited in local herbaria at Agricultural Research Company of Pernambuco and Federal University of Pernambuco (IPA and UFP, respectively).
A total of 147 species were studied (including 24 trees, 62 shrubs, 35 herbs, 21 vines and five epiphytes) distributed in 34 families, and 91 genera (see Appendix), which represents a small section of the number of species registered in the Caatinga (Sampaio et al., 2002; Leal et al., 2003).
Voucher specimens are deposited in the Herbarium UFP.
Floral traits, rewards and pollination systems
The pollination system of 142 species (excluding five which do not fit in a specific pollination system) was first inferred by floral attributes and syndromes (sensu Faegri and Pijl, 1979), and for the majority of the species (99, or 69·7 %) it was confirmed by direct observations in the field. In these cases the time of observation for visitors/pollinators varied from 2–4 h up to more than 100 h. Independently of the number of hours, it was always at least the minimum time required in order to allow us to distinguish between the behaviour of a simple visitor and a pollinator. With regard to the remaining 43 species studied: (a) 12 (8·5 %), in spite of a lack of field observations, are without doubt pollinated by bees; they belong to genera largely known to be bee-pollinated whose species offer pollen in poricidal anthers (four Solanum spp. and four Senna spp.) or oil (four Malpighiaceae spp.; see Appendix); and (b) 31 species (21·8%) were studied from specimens deposited in herbaria, and literature sources (see Andrade-Lima, 1989; Machado, 1996; Machado and Lopes, 2002; and references therein). Field trips occurred five to six times per year, each one lasting 3–5 days, from January 1994 to September 2002.
For each species, floral attributes were recorded as form, size, colour and floral rewards. For some species, observations were made related to the floral biology, including the behaviour of the visitors and pollinators. During the field studies, flowers and buds were fixed in ethanol (70 %) and/or FAA (50 %).
Flowers were classified according to floral types (‘structural blossom classes’) modified from Faegri and Pijl (1979). Eight floral types were considered: (1) tube; (2) gullet; (3) dish; (4) brush; (5) flag; (6) bell-funnel; (7) chamber; and (8) inconspicuous (<4 mm). The flower measurements (length and width) were taken from approx. 10–20 flowers per species. Flowers were classified as (1) small, ≤ 10 mm; (2) medium, >10 ≤ 20 mm; (3) large, >20 ≤ 30 mm; and (4) very large, >30 mm. Seven categories of flower colours were considered with regard to the main, most conspicuous, colour: (1) white; (2) red; (3) greenish (including beige, cream); (4) yellow; (5) orange; (6) lilac/violet (including blue); and (7) rose (light and pink).
Pollination units and the organization of the flowers and inflorescences were characterized according to Ramirez et al. (1990): (1) individual, when each flower is visited/explored individually; (2) collective, when the visitors explore and contact simultaneously with more than one flower; and (3) intermediate, when the visits are individual or collective according with the size and behaviour of the visitor/pollinator.
Five classes of floral resources were considered: (1) pollen; (2) nectar; (3) oil; (4) resin; and (5) pollen/nectar. In each class (excepting the last) only the main used resource was considered.
The species were also grouped into guilds according to the main pollinator vector: (1) wind; (2) medium-large bees (≥ 12 mm); (3) small bees (<12 mm); (4) wasps; (5) butterflies; (6) moths; (7) hawkmoths; (8) flies; (9) beetles; (10) diverse small insects; (11) hummingbirds; and (12) bats.
Statistical analysis
Analysis of the frequencies of the floral traits and pollination systems were tested by the G-test using the software BioEstat 2.0 (Ayres et al., 2000).
RESULTS AND DISCUSSION
Floral traits, rewards and pollination systems
The floral attributes varied largely with respect to colour, types, symmetry and size.
Colour
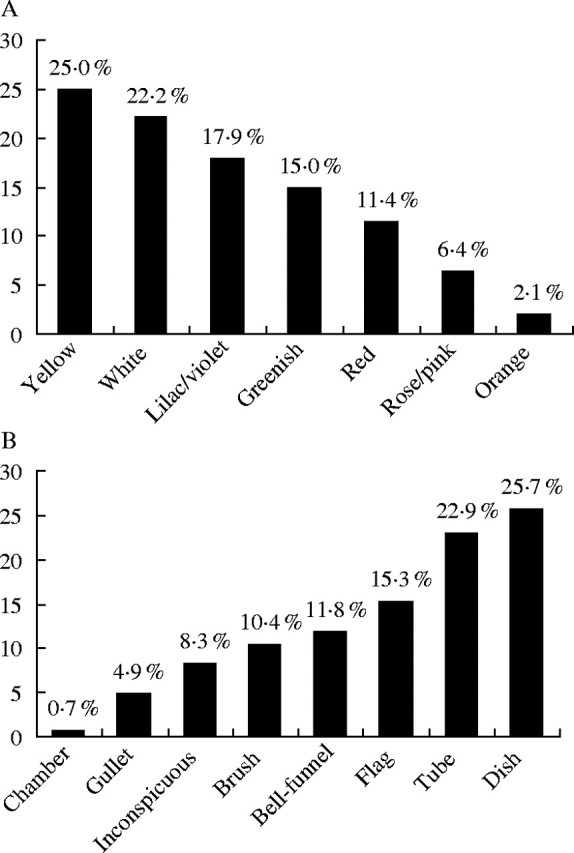
A high proportion of species with colourful flowers were observed: 62·8 % (including red, yellow, orange, lilac/violet and rose) compared with 37·2 % of species with pale flowers (white and greenish, including cream and beige). Yellow flowers were the most frequent (25·0 %), followed by white (22·2 %) (Fig. 2A). In contrast to these results, flowers of pale colours have been reported to be prevalent for savanna communities in Brazil (Table 1), for Venezuelan shrubland (Table 1) and also for humid forests (Mantovani and Martins, 1988; Silva et al., 1997).
Fig. 2.
Frequencies of (A) the floral colours and (B) types in Caatinga vegetation, northeastern Brazil.
Table 1.
Frequency of colour categories in diverse neotropical ecosystems, as number of species (%)
| Floral colours* |
Dry forest – Caatinga (this study) |
Dry forest – Cerrado (Silberbauer-Gottsberger and Gottsberger, 1988)1 |
Dry forest – Cerrado (Oliveira and Gibbs, 2000) |
Shrubland (Ramirez et al., 1990)1 |
Restinga (Ormond et al., 1993)2 |
|---|---|---|---|---|---|
| Yellow | 35 (25·0) | 56 (23·2) | 10 (17·0) | 6 (10·9) | 13 (9·6) |
| Orange | 3 (2·1) | – | 2 (3·4) | – | – |
| Greenish | 21 (15·0) | 63 (26·1) | 11 (18·6) | 9 (16·4) | 37 (26.0) |
| Lilac/violet | 25 (17·9) | 46 (19·1) | 4 (6·8) | 10 (18·2) | 25 (17.8) |
| Rose | 9 (6·4) | – | 3 (5·1) | – | – |
| Red | 16 (11·4) | 10 (4·2) | – | 8 (14·5) | 13 (8·9) |
| White | 31 (22·2) | 66 (27·4) | 29 (49·1) | 22 (40·0) | 53 (37·7) |
Greenish including beige, cream; lilac/violet including blue and lilac-reddish; rose, including light rose and pink.
Red including orange and rose.
Data for nectariferous species only.
Types and symmetry
A great variation of floral types was observed with a predominance of dish flowers (25·7 %), followed by tube (22·9 %) and flag types (15·3 %) (Fig. 2B). The high percentage of flag flowers was due to the high number of Leguminosae species.
The high frequency of tube and flag flowers contributed greatly to the high frequency of flowers whose floral reward is not easily available to visitors, which corresponded to 43·1 % of the species studied (represented by tube, gullet and flag flower types).
Actinomorphic flowers were found in the majority of the species (61·7 %), mainly due to tube and dish flowers (together with chamber, brush and inconspicuous types), with the remaining 38·3 % corresponding to zygomorphic species. Ramirez et al. (1990) also registered a high frequency of species with radial flowers (54·6 %), in comparison to 12·7 % with irregular and 32·7 % with bilateral flowers.
Sizes
Most of the species (54·1 %) had large (11·1 %) to very large flowers (43·0 %). Species with small and medium flowers were almost equally represented (23·7 and 22·2 %, respectively). Pseudobombax marginatum (Bombacaceae) and Pilosocereus spp. (Cactaceae) showed the largest flowers, whereas Thiloa glaucocarpa (Combretaceae) and Maprounea aff. guianensis (Euphorbiaceae) showed the smallest ones.
The size of the flowers is generally associated with the size of the respective pollinator (Opler, 1980). Some studies have shown correlations between the size of the floral tube and the size of the insects (Lindsey and Bell, 1985), the length of the proboscis (Real, 1983) or the length of the beak of the hummingbirds (Kodric-Brown et al., 1984). With few exceptions, short-tube flowers, together with dish, brush and inconspicuous flowers, allow access to a great diversity of pollinators (Faegri and Pijl, 1979).
Bawa et al. (1985) reported that flowers with pale colours visited by small diverse insects were found to be smaller than 10 mm. Among the species we studied, small flowers were found to be pollinated by diverse small insects (sensu Bawa and Opler, 1975; Bawa et al., 1985). However, when small flowers are organized in dense inflorescences it allows visits by medium-large bees, and also by bats and hummingbirds. For this reason, the generalist pollination system should not be inferred based only on the size of the flowers. Examples of species with small flowers which are, nonetheless, broadly attractive due to their organization in dense inflorescences are found in many Leguminosae, such as Anadenanthera colubrina, Mimosa tenuiflora, Acacia farnesiana, and Parapiptadenia zehntneri, as well as in other families such as Combretaceae (Combretum hilarianum and C. pisonioides), and Amaranthaceae (Gomphrena vaga).
Some relationships were observed between the size of the flowers and some colour classes. White and greenish flowers (including beige and cream) were generally smaller and tended to form collective units. This was also observed by Ramirez et al. (1990) in a tropical rain forest in Venezuela. In contrast, individually organized flowers were represented in practically all classes of colour and sizes, also corroborating what was found by Ramirez et al. (1990).
Pollination units
The individual pollination unit was the most representative, recorded in about 80·7 % of the species, compared with 14·5 % of collectivist and 4·8 % of intermediary types. Ramirez et al. (1990) also found the individual pollination unit as the most frequent in a tropical shrubby community in Venezuela. However, while the intermediate type was the least frequent in the present study, Ramirez et al. (1990) found a high percentage of species with this kind of pollination unit (27·3 %), with collectivists being the least common (9·1 %).
Floral resources
An interesting diversification of floral rewards was observed in the Caatinga vegetation, including very elaborate and unusual resources such as resin and oil. Nectar was the most frequent resource (Fig. 3A) at species (71·5 %), genus or family levels, similar to other studies in different ecosystems (Table 3; see also Percival, 1974; Silva et al., 1997). This is related to the high percentage of species pollinated by nectar-seeking insects together with the ornithophilous and chiropterophilous species. Pollen flowers were registered in approx. 15·3 % of the species, followed by oil (9·0 %), nectar and pollen (2·8 %) and resin (1·4 %) (Fig. 3A). Pollen flowers are mainly found in species of Leguminosae (Senna, Chamaecrista) and Solanaceae (Solanum). Pollen as a floral reward is offered mainly by species with poricidal anthers, pollinated by bees that vibrate during their visits (see Buchmann, 1983). This kind of anther dehiscence was found to occur in 11·0 % of all species investigated (the remaining 89 % of the species have longitudinal anther dehiscence), and all of them are pollinated by bees (Table 2). A higher frequency of species with poricidal anthers (30·9 %) was recorded by Ramirez et al. (1990), mainly due to the great number of species of Melastomataceae occurring at their study site, together with Ericaceae and Ochnaceae species.
Fig. 3.
Frequencies of (A) the floral rewards and (B) pollination systems in Caatinga vegetation, northeastern Brazil.
Table 3.
Frequency of floral reward categories in diverse neotropical ecosystems, as number of species (%)
| Floral rewards |
Dry forest – Caatinga (this study) |
Dry forest – Cerrado (Silberbauer-Gottsberger and Gottsberger, 1988) |
Dry forest – Cerrado (Oliveira and Gibbs, 2000) |
Shrubland (Ramirez et al., 1990) |
Restinga (Ormond et al., 1993)* |
|---|---|---|---|---|---|
| Nectar | 103 (71·5) | 128 (45·9) | 40 (70·2) | 20 (40·8) | 141 (62·0) |
| Pollen | 22 (15·3) | 46 (16·5) | 11 (19·3) | 13 (26·5) | – |
| Nectar + pollen | 4 (2·8) | 49 (17·6) | 3 (5·3) | 14 (28·6) | – |
| Oil | 13 (9·0) | 10 (3·6) | 2 (3·5) | – | – |
| Resin | 2 (1·4) | – | – | 2 (4·1) | – |
| Floral parts | 0 | 6 (2·2) | – | – | – |
| Without reward | 0 | 40 (14·3) | – | – | – |
| Pollen + Petals | 0 | – | 1 (1·7) | – | – |
| Total species | 144 | 269 | 57 | 49 | 228 |
Data for nectariferous species only.
Table 2.
Number of species of each pollination system with respect to floral traits
| Pollination systems |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Floral traits |
Wind |
Beetles |
Wasps |
Moths |
Butterflies |
Hawkmoths (Sphingids) |
Hummingbirds |
Diverse small insects |
Bats |
Medium-large bees |
Small bees |
|||||||||||
| Floral Symmetry | ||||||||||||||||||||||
| Actinomorphic | 3 | 1 | 1 | 2 | 4 | 10 | 13 | 18 | 13 | 22 | 17 | |||||||||||
| Zygomorphic | – | – | 1 | – | 2 | 1 | 10 | – | 5 | 31 | 5 | |||||||||||
| Floral reward | ||||||||||||||||||||||
| Nectar | – | – | 2 | 2 | 6 | 11 | 23 | 17 | 20 | 19 | 8 | |||||||||||
| Resin | – | – | – | – | – | – | – | – | – | 2 | 1 | |||||||||||
| Oil | – | – | – | – | – | – | – | – | – | 12 | 1 | |||||||||||
| Pollen | – | 1 | – | – | – | – | – | – | – | 18 | 9 | |||||||||||
| Nectar/pollen | – | – | – | – | – | – | – | 2 | – | – | 2 | |||||||||||
| Floral shape | ||||||||||||||||||||||
| Bell-funnel | – | – | – | – | – | 2 | – | – | 7 | 7 | 2 | |||||||||||
| Tube | – | – | – | 1 | 5 | 3 | 19 | – | 1 | 2 | 2 | |||||||||||
| Dish | 3 | – | 2 | – | – | 1 | 1 | 6 | 2 | 20 | 10 | |||||||||||
| Pseudanthus | – | – | – | – | – | – | – | – | – | 1 | 1 | |||||||||||
| Flag | – | – | – | – | – | – | 2 | – | 2 | 17 | 2 | |||||||||||
| Gullet | – | – | – | – | – | – | – | – | 1 | 4 | 2 | |||||||||||
| Brush | – | – | – | 1 | 1 | 5 | – | 3 | 7 | 1 | 2 | |||||||||||
| Inconspicuous | – | – | – | – | – | – | – | 10 | – | – | 1 | |||||||||||
| Chamber | – | 1 | – | – | – | – | – | – | – | – | – | |||||||||||
| Anther dehisc. | ||||||||||||||||||||||
| Longitudinal | 3 | 1 | 2 | 2 | 6 | 11 | 23 | 18 | 20 | 37 | 16 | |||||||||||
| Poricide | – | – | – | – | – | – | – | – | – | 16 | 6 | |||||||||||
| Floral size | ||||||||||||||||||||||
| ≤10 mm | 3 | – | 1 | 1 | 4 | – | 1 | 17 | 1 | 2 | 5 | |||||||||||
| >10 x ≤20 mm | – | 1 | – | 1 | 2 | – | 6 | 1 | 1 | 12 | 4 | |||||||||||
| >20 x ≤30 mm | – | – | – | – | – | 2 | 4 | – | 2 | 7 | 1 | |||||||||||
| >30 mm | – | – | 1 | – | – | 8 | 11 | 1 | 13 | 25 | 10 | |||||||||||
| Floral colour | ||||||||||||||||||||||
| Lilac | – | – | – | – | 1 | – | 2 | 1 | – | 15 | 8 | |||||||||||
| Yellow | – | – | – | – | 1 | 1 | 1 | 5 | 3 | 21 | 5 | |||||||||||
| White | – | – | 1 | 1 | 1 | 7 | – | 2 | 13 | 6 | 8 | |||||||||||
| Greenish-cream | 3 | – | 1 | – | – | 3 | 1 | 9 | 4 | 3 | – | |||||||||||
| Red | – | – | – | 1 | – | – | 14 | 1 | – | 1 | – | |||||||||||
| Rose | – | – | – | – | 2 | – | 4 | 1 | – | 1 | 1 | |||||||||||
| Orange | – | 1 | – | – | 1 | – | 1 | – | – | – | – | |||||||||||
| Sexual systems | ||||||||||||||||||||||
| Hermaphrodite – homostylous | – | 1 | 1 | 2 | 5 | 11 | 21 | 7 | 20 | 41 | 12 | |||||||||||
| Hermaphrodite – heterostylous | – | – | – | – | – | – | 1 | 1 | – | 1 | 3 | |||||||||||
| Dioecious | – | – | – | – | – | – | – | 2 | – | 1 | – | |||||||||||
| Monoecious | 3 | – | 1 | – | 1 | – | 1 | 7 | – | 2 | 1 | |||||||||||
| Andromonoecious | – | – | – | – | – | – | – | 1 | – | 6 | 6 | |||||||||||
| Poll. Unit | ||||||||||||||||||||||
| Flower | – | 1 | 1 | 1 | 2 | 11 | 23 | 3 | 19 | 49 | 18 | |||||||||||
| Inflorescence (collectivist ) | – | – | 1 | – | 4 | – | – | 11 | 1 | 2 | 4 | |||||||||||
| Flower/Inflorescence (intermediate) | 3 | – | – | 1 | – | – | – | 4 | – | 1 | – | |||||||||||
| Species of each pollination system* | 3 | 1 | 2 | 2 | 6 | 11 | 23 | 19 | 20 | 66 | ||||||||||||
n = 142 species (99 with direct observation in the field for pollinators); 5 species which do not fit in a specific pollination system were excluded; 7 species pollinated by hawkmoths and bats, were included in both pollination systems; 3 species pollinated by wind and diverse small insects were included in both systems; 1 species, pollinated by bees and hummingbirds, was included in both systems; 9 species pollinated by small and medium-large bees were included in both.
In the present study, the percentage of flowers offering both pollen and nectar as a floral reward (pollen in this category being intentionally collected) was lower (2·8 %) than that recorded in other tropical shrubby communities (17·6 %, Silberbauer-Gottsberger and Gottsberger, 1988; 28·6 %, Ramirez et al., 1990; Table 3).
Floral oils were mainly restricted to plant species of the herbaceous and shrubby strata, such as Angelonia spp. (Scrophulariaceae) (Vogel and Machado, 1991; Machado et al., 2002), Byrsonima spp. (Malpighiaceae) and Krameria tomentosa (Krameriaceae) (Machado et al. 1997b). These species constitute an important food source for the larvae of anthophorid bees (Vogel, 1974) and are thus essential for the maintenance of this bee guild in the community.
In this study, floral resin was found to be offered by flowers of Clusia nemorosa and Dalechampia sp. Among angiosperms, resin is reported as a floral reward only in these two genera plus Clusiella, which are mostly distributed in humid areas (cf. Armbruster, 1984; Bittrich and Amaral, 1997; Lopes and Machado, 1998). Thus, the presence of any species with resin flowers was surprising, due to their rarity and distribution.
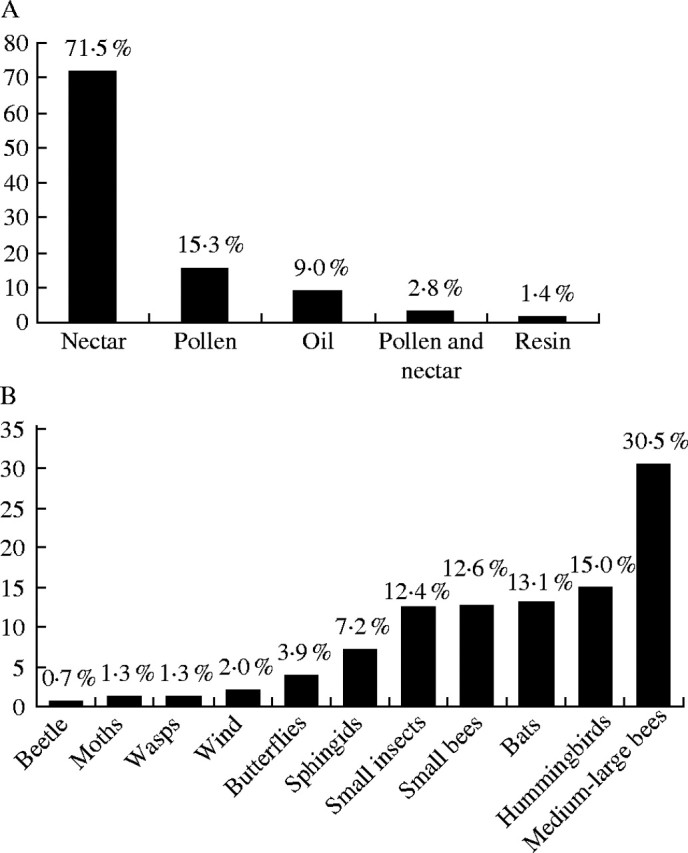
Pollination systems
Insect pollination was the most frequent, occurring in approx. 69·9 % of the studied species, followed by hummingbird (15·0 %) and bat pollination (13·1 %) (Fig. 3B; Table 4). Among the entomophilous species, 61·7 % were considered to be melittophilous, with the remaining 38·3 % pollinated by diverse other groups of insects. Of the 34 families investigated, the only one lacking any entomophilous species was Tiliaceae (here represented only by one chiropterophilous species of Luehea). At the community level, the proportion of species pollinated by bees is the largest (43·1 %), similar to other tropical forest ecosystems (Table 4). The frequency of species pollinated by medium-large and small bees also agreed with those observed in other tropical ecosystems, where pollination by medium-large bees is the most representative (Table 4).
Table 4.
Percentage of pollination systems in this study and in other tropical communities
| Pollination system |
Dry forest – Caatinga (this study) |
Dry forest – Cerrado (Silberbauer-Gottsberger and Gottsberger, 1988) |
Dry forest –Cerrado (Oliveira and Gibbs, 2000) |
Coastal vegetation – Restinga (Ormond et al., 1993)1 |
Shrubland (Ramirez, 1989) |
Rain forest (Bawa et al. 1985) |
Rain forest (Kress and Beach, 1994) |
Dipterocarp forest (Kato, 1996) |
|---|---|---|---|---|---|---|---|---|
| Wind | 2·0 | 13·6 | 0·0 | – | 8·2 | 2·5 | 2·5 | 0·0 |
| Beetles | 0·7 | 2·8 | 2·0 | – | 2·7 | 7·3 | 12·7 | 2·4 |
| Wasps | 1·3 | – | – | – | – | 4·3 | 2·5 | 2·4 |
| Moths | 1·3 | 2·22 | 12·0 | 29·82 | 10·92 | 7·9 | 8·0 | 2·4 |
| Butterflies | 3·9 | 0·0 | 4·9 | 4·3 | 2·4 | |||
| Sphingids | 7·2 | 2·2 | – | 4·5 | – | 8·0 | – | – |
| Hummingbirds | 15·0 | 1·8 | 2·0 | 5·4 | 12·3 | 4·3 | 14·9 | – |
| Sunbird | 0·0 | – | – | – | – | – | – | 9·8 |
| Diverse small insects | 12·4 | – | 49·0 | – | – | 15·8 | 11·2 | 2·4 |
| Bats | 13·1 | 1·8 | 3·0 | 2·1 | – | 3·0 | 3·6 | 0·0 |
| Medium-large bees | 30·5 | 65·23 | 32·0 | 40·83 | 56·23 | 27·5 | 24·3 | 26·7 |
| Small bees | 12·6 | – | 14·0 | 14·1 | 44·0 | |||
| Flies | 0·0 | 10·4 | – | 17·8 | 9·6 | – | 1·8 | 7·3 |
| Thrips | 0·0 | – | – | – | – | 0·6 | – | – |
Data for nectariferous species only
sum of percentages of moth and butterfly pollination systems
sum of percentages of medium-large bees and small bees pollination systems.
With respect to pollination systems other than entomophilous, we found a high percentage of ornithophilous and chiropterophilous species in comparison with Bawa et al. (1985), Silberbauer-Gottsberger and Gottsberger (1988) and Oliveira and Gibbs (2000) (Table 4). The level of ornithophily was, however, similar to some studies (Feinsinger, 1983; Linhart et al., 1987; Ramirez, 1989; Kress and Beach, 1994; Table 4). In humid tropical forests at least, ornithophily has been mentioned in the literature as occurring in approx. 10–15 % of the species of a local flora (Feinsinger, 1983; Linhart et al., 1987; Kress and Beach, 1994; Buzato et al., 2000). The percentage of bat-pollinated flowers was greater than in other studies (Table 4).
The most representative families with ornithophilous species at our sites were: Acanthaceae, Bromeliaceae, Cactaceae, Leguminosae, Passifloraceae, and Sterculiaceae. Eight species of hummingbird were recorded: Amazilia fimbriata, A. lactea, A. versicolor, Chlorostilbon aureoventris, Chrysolampis mosquitus, Eupetomena macroura, Hylocharis sapphirina and Phaethornis gounellei. These birds forage on different plant species, some of them visiting flowers that do not have typical attributes of the ornithophilous syndrome, such as the melittophilous flowers of Melochia tomentosa (Sterculiaceae) and Lonchocarpus aff. campestris (Leguminosae). Except for Phaethornis gounellei, the other hummingbird species are of the subfamily Trochilinae, which is normally associated with opportunistic behaviour (Des Granges, 1978; Sick, 1997; Buzato et al., 2000).
In contrast to generalist behaviour, hummingbirds were observed in the field to act as effective and exclusive pollinators in Ruellia asperula (Acanthaceae), species of Cactaceae (Opuntia spp. and Melocactus spp.) and Bromeliaceae (Portea leptantha, Billbergia porteana and others), Passiflora luetzelburgii (Passifloraceae), Periandra coccinea (Leguminosae), Helicteres spp. (Sterculiaceae).
Chiropterophilous species were found in Acanthaceae, Bombacaceae, Bromeliaceae, Cactaceae, Capparaceae, Convolvulaceae, Leguminosae, Passifloraceae and Tiliaceae (Machado et al., unpubl.). These same families have other chiropterophilous species in many different ecosystems (e.g. Vogel, 1968; Dobat and Peikert-Holle, 1985; Sazima et al., 1999).
Only one species (0·7 %), belonging to the Annonaceae family, was found to be cantharophilous. Percentages of beetle pollination in the literature vary from 2 % (Oliveira and Gibbs, 2000) to 12·7 % (Kress and Beach, 1994)(Table 4).
Anemophilous species were also not numerous (Table 4), corresponding to 2 % of the studied species. Wind pollination has been cited by Kress and Beach (1994) as including 2·5 % of the flora of tropical forests. Bawa et al. (1985) also recorded 2·5 % of wind-pollinated species among tropical lowland rain forest trees. Oliveira and Gibbs (2000) did not record any species pollinated by wind among woody plants in a Brazilian savanna. However, Silberbauer-Gottsberger and Gottsberger (1988), studying a different Brazilian savanna community, found wind pollination to occur in 13·6 % of the species (Table 4).
Pollination systems × floral traits
Zygomorphic flowers were mostly found among hummingbird- and medium-large bee-pollinated species (Table 2) and, in fact, zygomorphy is largely known as a visual attractant for some groups of pollinators such as bees and hummingbirds (Faegri and Pijl, 1979; Endress, 1994; Proctor et al., 1996). Conversely, flowers pollinated by wind and diverse small insects, for example, were 100 % actinomorphic. Nocturnal flowers, pollinated by bats and hawkmoths mainly orientated by sonar (von Helversen, 1993) and olfactory cues, respectively were mostly actinomorphic, too.
With respect to floral rewards, resin and oil were always related to bee-flowers, as well as pollen-flowers; the latter, with the exception of one beetle-pollinated species, were also associated with bee pollination. In fact resin and oil are strictly related to bee-flowers (Vogel, 1974; Armbruster, 1984). Flowers offering both nectar and pollen were, as expected, pollinated by diverse small insects, including small bees. Nectar, known as the most widespread floral resource (e.g. Faegri and Pijl, 1979; Endress, 1994; Proctor et al., 1996), was recorded in all classes of pollination systems. In most of the cases it was the only offered reward, as for hawkmoth-, bat- and hummingbird-pollinated species.
Associations were observed between floral shape and the pollination system. The great majority of hummingbird- and butterfly-pollinated flowers were of the tube type; most of the diverse small insect-pollinated flowers were inconspicuous; bat-pollinated species had mainly bell-funnel or brush flowers, and bee-pollinated flowers were mainly of the dish or flag type.
Regarding floral size and colour, the frequency of small flowers visited by diverse small insects was significantly different from the frequencies of medium and large flowers visited by this group of pollinators (G = 26·19; P < 0·001, DF = 2; Table 2). Significant differences were also observed in the frequency of greenish-cream and yellow flowers visited by diverse small insects compared with those of other colour categories (G = 14·61; P = 0·012; DF = 5; Table 2). These small insects showed a preference for grouped flowers, forming collectivist pollination units, when compared with the other kinds of pollination units (G = 5·93; P < 0·05; DF = 2; Table 2).
Studies in lowland tropical forests show that the frequencies of flower colours by themselves are not significantly related to the pollination systems (Momose et al., 1998; see also Johnson and Steiner, 2000 for a review). In fact if the frequency of any single floral attribute is used to study the frequencies of pollination systems, then the influence of other attributes is neglected. Nonetheless, it is apparent that some pollinators preferentially visit flowers of some colours (or have preference for some other attribute as mentioned above). For example, our results show that among the species visited by hawkmoths and bats, white flowers predominated, a result also found in other studies (Muchhala and Jarrín-V, 2002). Similar relationships are observed for hummingbirds and red flowers, and medium-large bees and yellow flowers, although these colours are visited by other animals, too (Table 2).
CONCLUDING REMARKS
The range of pollination characters in Caatinga species is similar to other tropical forests. Specialized pollination mechanisms were found to be common in the Caatinga, although more generalization might have been expected for an open vegetation with extreme climatic conditions (low and irregular precipitation). Examples are the existence of a relatively high percentage (9·0 %) of oil flower species; the high percentage of vertebrate pollination (28·1 %) and the also great number of species (43·1 %) with hidden floral rewards (represented by the types tube, gullet and flag).
To avoid misinterpretation and to check if our values for pollination systems really reflect a typical Caatinga community, 19 species occurring only in Buíque, a special type of Caatinga (Rodal et al., 1998) were removed from the analyses. The differences in the results were not found to be significant (pollination systems G = 0·42, NS; floral colours G = 7·57, NS; floral rewards G = 4·2, NS), showing the similarity of the floral traits and pollination systems between the different physiognomies of the Caatinga. The observed floral features obviously reflect the characteristic plant families and their adaptations, and are not a product of selective forces.
Some preconceptions about Caatinga biodiversity, such as ‘the Caatinga biome is homogeneous and poor in species and endemisms’, are proving to be false on closer study (MMA, 2002). Our results show the diversity and uniqueness of this vegetation type in the reproductive biology of its plants, a subject of critical importance in the urgent task of conservation.
Appendix
Families and genera of the species studied. Species followed by an asterisk were observed in the field for pollinators.
Acanthaceae (2 genera/4 species)
Harpochilus nessianus Mart.*
Ruellia asperula (Nees) Lindau*
Ruellia aff. paniculata L.*
Ruellia sp.*
Amaranthaceae (1/1)
Gomphrena vaga Mart.
Anacardiaceae (3/3)
Myracrodruon urundeuva Allemão*
Schinopsis brasiliensis Engl.
Spondias tuberosa Arruda*
Annonaceae (1/1)
Rollinia leptopetala R.E. Fries*
Apocynaceae (3/4)
Aspidosperma pyrifolium Mart.*
Allamanda blancheti A. DC.
Allamanda sp.
Mandevilla tenuifolia (Mikan) Woodson
Bignoniaceae (2/2)
Tabebuia impetiginosa (Mart. ex DC.) Standl.
Anemopaegma sp.
Bombacaceae (2/2)
Pseudobombax marginatum (A. St.-Hil.) A.Robyns*
Ceiba glaziovii (Kuntze) K.Schum.*
Boraginaceae (1/2)
Cordia globosa (Jacq.) Kunth*
C. leucocephala Moric.*
Bromeliaceae (6/8)
Dyckia pernambucana L.B.Sm.*
Billbergia porteana Brongn.*
Encholirium spectabile Mart. ex Schult.f.*
Neoglaziovia variegata (Arr.Cam.) Mez.*
Portea leptantha Harms*
Tillandsia gardneri Lindl.*
T. loliacea Mart. ex Schult.
T. streptocarpa Baker
Burseraceae (1/1)
Commiphora leptophloeos (Mart.) J.B. Gillett
Cactaceae (5/11)
Cereus jamacaru DC.*
Harrisia adscendens (Gürke) Britton & Rose
Melocactus bahiensis (Br. Et Rose) Werderm.*
M. zehntneri (Britton & Rose) Luetzelburg*
Opuntia inamoena K. Schum.*
O. palmadora Britton & Rose*
Pilosocereus catingicola (Gürke) Byles & G.D. Rowley*
P. chrysostele (Vaupel) Byles & G.D. Rowley*
P. gounellei (F.A.C.Weber) Byles & G.D. Rowley*
P. pachycladus (Werderm.) Byles & G.D. Rowley*
P. tuberculatus (Werderm.) Byles & G.D. Rowley*
Capparaceae (1/4)
Capparis hastata Jacq.*
C. flexuosa (L.) L.*
C. jacobinae Moric. ex Eichler*
C. yco (Mart.) Eichler
Clusiaceae (1/1)
Clusia nemorosa G. Mey.*
Combretaceae (2/4)
Combretum hilarianum D. Dietr.
C. leprosum Mart.*
C. pisonioides Taub.*
Thiloa glaucocarpa (Mart.) Eichler
Convolvulaceae (4/7)
Evolvulus sp.
Ipomoea acuminata (Vahl) Roem. & Schult.
Ipomoea sp.1
Ipomoea sp.2
Jacquemontia densiflora (Meissn.) Hall.
Merremia aegyptia L.Urban*
Merremia sp.
Euphorbiaceae (8/12)
Acalypha multicaulis Müll. Arg.
Cnidoscolus urens (L.) Arthur*
Croton argyrophylloides Müll. Arg.*
C. sonderianus Müll. Arg.*
Croton sp.
Dalechampia sp.*
Euphorbia comosa Vell.
Jatropha mollissima (Pohl.) Baill.*
J. mutabilis (Pohl.) Baill.*
J. ribifolia (Pohl.) Baill.*
Manihot cf. pseudoglaziovii Pax & K. Hoffm.
Maprounea aff. guianensis Aubl.
Krameriaceae (1/1)
Krameria tomentosa A. St.-Hil.*
Lamiaceae (1/1)
Hyptis martiusii Benth.*
Leguminosae (15/29)
Acacia farnesiana (L.) Willd.*
Amburana cearensis (Allemão) A.C. Sm.
Anadenanthera colubrina var. cebil (Griseb.) Altschul.
Bauhinia acuruana Moric.*
B. cheilantha (Bong.) Vogel ex Steud.*
B. pentandra (Bong.) Vogel ex Steud.*
Caesalpinia ferrea Mart. ex Tul.*
C. pyramidalis Tul.*
Calliandra aeschynomenoides Benth.*
Chamaecrista cytisoides (Collad.) Irwin & Barneby*
Chamaecrista ramosa (Vogel) Irwin & Barneby*
Chamaecrista sp.1*
Cratylia mollis Mart. ex Benth.*
Erythrina velutina Willd.*
Lonchocarpus aff. campestris Benth.*
Mimosa lewisii Barneby*
M. tenuiflora (Willd.) Poir.
Parapiptadenia zehntneri (Harms) M.P. Lima & H.C. Lima*
Periandra coccinea (Schrad.) Benth.*
Senna acuruensis (Benth.) Irwin & Barneby*
S. angulata (Vogel) Irwin & Barneby*
S. chrysocarpa (Desv.) Irwin & Barneby*
S. macranthera (Collad.) Irwin & Barneby
S. martiana (Benth.) Irwin & Barneby*
S. rizzini Irwin & Barneby
S. spectabilis (DC.) var. excelsa (Schrad.) Irwin & Barneby*
S. splendida (Vogel) Irwin & Barneby
S. trachypus (Benth.) Irwin & Barneby
Zornia sericea Moric.
Malpighiaceae (5/7)
Banisteriopsis schizoptera (A. Juss.) B. Gates
Byrsonima gardneriana A. Juss.*
Byrsonima vacciniaefolia A. Juss.*
Byrsonima sp.
Janusia anisandra (Juss.) Griseb.
Heteropteris sp.
Stigmaphyllum paralias A. Juss.*
Malvaceae (4/5)
Bakeridesia pickelii Monteiro*
Herissantia tiubae (K.Sch.) Briz.*
Pavonia humifusa A. St.-Hil.
P. martii Mart. ex Colla*
Sida sp.*
Orchidaceae (2/2)
Cyrtopodium intermedium Brade*
Indet. sp. 1*
Passifloraceae (1/4)
Passiflora foetida L.*
P. luetzelburgii Harms*
Passiflora sp.1*
Passiflora sp.2
Rhamnaceae (1/1)
Ziziphus joazeiro Mart.*
Rubiaceae (2/2)
Coutarea hexandra (Jacq.) K. Schum.
Tocoyena formosa (Cham. & Schltdl.) K. Schum.*
Sapindaceae (2/2)
Allophylos quercifolius (Mart.) Radlk.
Serjania comata Radlk.*
Sapotaceae (1/1)
Bumelia sartorum Mart.*
Scrophulariaceae (3/7)
Angelonia bisaccata Benth.*
A. cornigera Hook.*
A. hirta Cham.*
A. hookeriana Gardn.*
A. pubescens Benth.*
Bacopa sp.*
Stemodia sp.*
Solanaceae (2/7)
Nicotiana tabacum L.
Solanum asperum Rich.
S. baturitense Huber*
S. crinitum Lam.
S. gardneri Sendtn.
S. paludosum Moric.*
S. variabile Mart.
Sterculiaceae (3/4)
Helicteres mollis K. Schum.*
H. velutina K. Schum.*
Melochia tomentosa L.*
Waltheria rotundifolia Schrank*
Tiliaceae (1/1)
Luehea sp.*
Turneraceae (1/2)
Turnera diffusa Willd. ex Schult.*
Turnera sp.
Verbenaceae (2/3)
Lantana camara L.*
Lippia gracilis Schau.*
L. schomburgkiana Schau.
Violaceae (1/1)
Hybanthus calceolaria (L.) G.K. Schulze.*
Acknowledgments
We are very grateful to Prof. S. Vogel (University of Vienna) for fruitful discussions during 15 years of collaboration and to Prof. P.E. Gibbs (University of St. Andrews, Scotland) and Prof. S. Bullock (University of San Diego, USA) for critically reading the manuscript and also for kindly improving the English. We thank the curators and taxonomists of the Herbaria UFP and IPA and the taxonomists Drs F. Agra (UFPB), G.S. Baracho (UFPE), L.P. Felix (UFPB), I.B. Loiola (UFRN), L. Paganucci (UEFS), R. Pereira (IPA), J. Semir (UNICAMP), J.A. Siqueira-Filho (UFPE), V. Souza (Esalq-USP) and D. Zappi (Kew Garden) for identifying most of the plant species. M.J.L. Santos (UFPE) is thanked for pleasant company and help in some field trips, and A.M. Santos (UFPE) for his assistance in the statistical analysis. The owners/administrators of the study sites are thanked for permission to carry out our studies within areas under their care. Two anonymous referees are acknowledged for their valuable suggestions. The UFPE, CNPq, CAPES, FACEPE, and FBPN-MacArthur Foundation are thanked for essential financial support.
LITERATURE CITED
- Aguiar J, Lacher T, Silva JMC. 2002. The Caatinga. In: Gil PR, ed., Wilderness – Earth's last wild places. Mexico: CEMEX, 174–181. [Google Scholar]
- Aizen MA, Feisinger P. 1994. Forest fragmentation, pollination, and plant reproduction in a chaco dry forest, Argentina. Ecology 75: 330–351. [Google Scholar]
- Andrade-Lima D. 1989.Plantas das Caatingas. Rio de Janeiro: Academia Brasileira de Ciências. [Google Scholar]
- Armbruster WS. 1984. The role of resin in angiosperm pollination: ecological and chemical considerations. American Journal of Botany 71: 1149–1160. [Google Scholar]
- Ayres M, Ayres-Junior M, Ayres DL, Santos AS. 2000. Bioestat 2.0: Aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém: Sociedade Civel Mamirauá, CNPq. [Google Scholar]
- Bawa KS. 1990. Plant–pollinator interactions in tropical rain forests. Annual Review of Ecology and Systematics 21: 399–422. [Google Scholar]
- Bawa KS, Opler PA. 1975. Dioecism in tropical forest trees. Evolution 29: 167–179. [DOI] [PubMed] [Google Scholar]
- Bawa KS, Bullock SH, Perry DR, Coville RE, Grayum MH. 1985. Reproductive biology of tropical lowland rain forest trees. II. Pollination systems. American Journal of Botany 72: 346–356. [Google Scholar]
- Bittrich V, Amaral MC. 1997. Floral biology of some Clusia species from Central America. Kew Bulletin 52: 617–635. [Google Scholar]
- Buchmann SL. 1983. Buzz pollination in Angiosperms. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand and Reinhold, 73–113. [Google Scholar]
- Buzato S, Sazima M, Sazima I. 2000. Hummingbird-pollinated floras at three Atlantic forest sites. Biotropica 32: 824–841. [Google Scholar]
- Chittka L, Spaethe J, Schmidt A, Hickelsberger A. 2001. Adaptation, constraint, and chance in the evolution of flower color and pollinator color vision. In: Chittka L, Thomson JD, eds. Cognitive ecology of pollination. Cambridge: Cambridge University Press, 106–126. [Google Scholar]
- Cruden RW. 1997. Implications of evolutionary theory to applied pollination ecology. Acta Horticulturae 437: 27–51. [Google Scholar]
- Dafni A, O'Toole C. 1994. Pollination syndromes in the Mediterranean: generalizations and peculiarities. In: Arianoutsou M, Groves RH, eds. Plant–animal interactions in Mediterranean-type ecosystems. Netherlands: Kluwer Academic Publishers, 125–135. [Google Scholar]
- Des Granges JL. 1978. Organization of a tropical nectar feeding bird guild in a variable environment. Living Bird 17: 199–236. [Google Scholar]
- Dobat K, Peikert-Holle T. 1985.Blüten und Fledermäuse. Frankfurt: Waldemar Kramer Verlag. [Google Scholar]
- Dressler RL. 1968. Observations on orchids and Euglossine bees in Panama and Costa Rica. Revista de Biologia Tropical 15: 143–183. [Google Scholar]
- Dressler RL. 1968. Pollination by Euglossine bees. Evolution 22: 202–210. [DOI] [PubMed] [Google Scholar]
- Egler WA. 1951. Contribuição ao estudo da Caatinga pernambucana. Revista Brasileira de Geografia 13: 577–590. [Google Scholar]
- Endress PK. 1994.Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press. [Google Scholar]
- Faegri K, van der Pijl L. 1979.The principles of pollination ecology, 3rd edition. Oxford: Pergamon. [Google Scholar]
- Feinsinger P. 1983. Coevolution and pollination. In: Futuyma DJ, Slatkin M, eds. Coevolution. Massachusetts: Sinauer Associates, 282–310. [Google Scholar]
- Feinsinger P, Colwell RK. 1978. Community organization among neotropical nectar-feeding birds. American Zoologist 18: 779–795. [Google Scholar]
- Ferraz EM, Rodal MJN, Sampaio EVSB, Pereira RCA. 1998. Composição florística em trechos de vegetação de Caatinga e brejo de altitude na região do vale do Pajeú, Pernambuco. Revista Brasileira de Botânica 21: 7–15. [Google Scholar]
- Figueiredo LS, Rodal MJN, Melo AL. 2000. Florística e fitossociologia de uma área de vegetação arbustiva caducifólia espinhosa no município de Buíque – Pernambuco. Naturalia 25: 205–224. [Google Scholar]
- Frankie GW, Haber WA, Opler PA, Bawa KS. 1983. Caracteristics and organization of the large bee pollination system in the Costa Rican dry forest. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold Company Inc., 411–447. [Google Scholar]
- Gegear RJ, Laverty TM. 2001. The effect of variation among floral traits on the flower constancy of pollinators. In: Chittka L, Thomson JD, eds. Cognitive ecology of pollination. Cambridge: Cambridge University Press, 1–20. [Google Scholar]
- Gil PR (ed.) 2002.Wilderness – Earth's last wild places. Mexico: CEMEX. [Google Scholar]
- Griz LMS, Machado IC. 2001. Fruiting phenology and seed dispersal syndromes in Caatinga, a tropical dry forest in the northeast of Brazil. Journal of Tropical Ecology 17: 303–321. [Google Scholar]
- Herrera CM. 1995. Floral traits and plant adaptation to insect pollinators: a devil's advocate approach. In: Lloyd DG, Barrett SCH, eds. Floral biology. New York: Chapman and Hall, 65–87. [Google Scholar]
- Hueck K. 1972. A região da Caatinga do Nordeste brasileiro. In: As florestas da América do Sul. São Paulo: Ed. Universidade de Brasília e Ed. Polígono S.A., 306–327. [Google Scholar]
- Johnson SD, Steiner KE. 2000. Generalization versus specialization in plant pollination systems. Tree 15: 140–143. [DOI] [PubMed] [Google Scholar]
- Kato M. 1996. Plant–pollinator interactions in the understory of a lowland mixed dipterocarp forest in Sarawak. American Journal of Botany 83: 732–743. [Google Scholar]
- Kearns CA, Inouye DW. 1997. Pollinators, flowering plants, and conservation biology. BioScience 47: 297–307. [Google Scholar]
- Kiill LHP, Drummond MA. 2001. Biologia floral e sistema reprodutivo de Gliricidia sepium (Jacq.) Steud. (Fabaceae- Papilionoidae) na região de Petrolina, Pernambuco. Ciência Rural 31: 597–601. [Google Scholar]
- Kiill LHP, Ranga NT. 2000. Biologia da polinização de Merremia aegyptia (L.) Urb. (Convolvulaceae) no Sertão de Pernambuco. Naturalia 25: 149–158. [Google Scholar]
- Kodric-Brown A, Brown J, Byers GS, Gori DF. 1984. Organization of a tropical island community of hummingbirds and flowers. Ecology 65: 1358–1368. [Google Scholar]
- Kress WJ, Beach JH. 1994. Flowering plant reproductive systems. In: McDade LA, Bawa KS, Hespenheide H, Hartshorn G, eds. La Selva: ecology and natural history of a neotropical rain forest. Chicago: University of Chicago Press, 161–182. [Google Scholar]
- Leal IR, Tabarelli M, Silva JMC. 2003.Ecologia e conservação da Caatinga. Recife: Editora Universitária –UFPE. [Google Scholar]
- Leppik EE. 1968. Directional trend of floral evolution. Acta Biotheoretica 18: 87–102. [Google Scholar]
- Lewis G, Gibbs P. 1999. Reproductive biology of Caesalpinia calycina and C. pluviosa (Leguminosae) of the Caatinga of north-eastern Brazil. Plant Systematics and Evolution 217: 43–53. [Google Scholar]
- Lindsey AH, Bell CR. 1985. Reproductive biology of Apiaceae. II. Cryptic specialization and floral evolution in Thaspium and Zizia American Journal of Botany 72: 231–247. [Google Scholar]
- Linhart YB, Busby WH, Beach JH, Feinsinger P. 1987. Forager behavior, pollen dispersal, and inbreeding in two species of hummingbird-pollinated plants. Evolution 41: 679–682. [DOI] [PubMed] [Google Scholar]
- Locatelli E, Machado IC. 1999. Comparative study of the floral biology in two ornithophilous species of Cactaceae: Melocactus zehntneri and Opuntia palmadora Bradleya 17: 75–85. [Google Scholar]
- Lopes AV, Machado IC. 1998. Floral biology and reproductive ecology of Clusia nemorosa (Clusiaceae) in northeastern Brazil. Plant Systematics and Evolution 213: 71–90. [Google Scholar]
- Machado ICS. 1990.Biologia floral de espécies de Caatinga no município de Alagoinha (PE). Tese de Doutorado. Campinas, Universidade Estadual de Campinas. [Google Scholar]
- Machado ICS. 1996. Biologia floral e fenologia. In: Sampaio EVSB, Mayo SJ, Barbosa MRV, eds. Pesquisa botânica nordestina: progresso e perspectivas. Recife: Editora Universitária da UFPE, 161–172. [Google Scholar]
- Machado IC, Lopes AV. 2002. A Polinização em ecossistemas de Pernambuco: uma revisão do estado atual do conhecimento In: Tabarelli M, Silva JMC, orgs. Diagnóstico da biodiversidade de pernambuco. Recife: Secretaria de Ciência Tecnologia e Meio-Ambiente, Fundação Joaquim Nabuco e Editora Massangana, 583–596. [Google Scholar]
- Machado ICS, Sazima M. 1995. Biologia da polinização e pilhagem por beija-flores em Ruellia asperula Lindau (Acanthaceae) na Caatinga de Pernambuco. Revista Brasileira de Botânica 18: 27–33. [Google Scholar]
- Machado ICS, Barros LM, Sampaio EVSB. 1997. Phenology of Caatinga species at Serra Talhada, PE, Northeastern Brazil. Biotropica 29: 57–68. [Google Scholar]
- Machado IC, Siqueira-Filho JA, Lopes AV, Vogel S. 1997. Organização e polinização das flores de óleo de Krameria tomentosa (Krameriaceae). In: Resumos do XLVIII Congresso Nacional de Botânica. Crato: Editora Universitária, 19. [Google Scholar]
- Machado IC, Vogel S, Lopes AV. 2002. Pollination of Angelonia cornigera Hook. (Scrophulariaceae) by long-legged, oil-collecting bees in NE Brazil. Plant Biology 4: 352–359. [Google Scholar]
- Mantovani W, Martins FR. 1988. Variacões fenológicas das espécies do cerrado da Reserva Biológica de Mogi Guaçu, Estado de São Paulo. Revista Brasileira de Botânica 11: 101–112. [Google Scholar]
- MMA. 2002. Avaliação e ações prioritárias para a conservação da biodiversidade da Caatinga. Brasília: Ministério do Meio Ambiente. [Google Scholar]
- Momose K, Yumoto T, Nagamitsu T, Kato M, Nagamasu H, Sakai S, Harrison RD, Itioka T, Hamid AA, Inoue T. 1998. Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. I. Characteristics of the plant-pollinator community in a lowland dipterocarp forest. American Journal of Botany 85: 1477–1501. [PubMed] [Google Scholar]
- Muchhala N, Jarrín-V P. 2002. Flower visitation by bats in Cloud forests of western Ecuador. Biotropica 34: 387–395. [Google Scholar]
- Murcia C. 1995. Forest fragmentation and the pollination of neotropical plants. In: Schelhas J, Greenberg R, eds. Forest patches in tropical landscapes. London: Island Press, 19–36. [Google Scholar]
- Murcia C. 2002. Ecología de la polinización. In: Guariguata MR, Kattan GH, eds. Ecología y conservación de Bosques neotropicales. Cartago: Ediciones LUR, 493–530. [Google Scholar]
- Oliveira PE, Gibbs PE. 2000. Reproductive biology of woody plants in a cerrado community of Central Brazil. Flora 195: 311–329. [Google Scholar]
- Ollerton J. 1996. Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant-pollinator systems. Journal of Ecology 84: 550–560. [Google Scholar]
- Opler PA. 1980. Nectar production in a tropical ecosystem. In: Bentley B, Elias T, eds. The biology of nectaries. New York: Columbia University Press, 30–79. [Google Scholar]
- Ormond WT, Pinheiro MCB, Lima HA, Correia MCR, Pimenta ML. 1993. Estudo das recompensas florais das plantas da restinga de Maricá – Itaipuaçu, RJ. I – Nectaríferas. Bradea 6: 179–195. [Google Scholar]
- Percival M. 1974. Floral ecology of coastal scrub in Southeast Jamaica. Biotropica 6: 104–129. [Google Scholar]
- Piedade-Kiill LH, Ranga NT. 2000. Biologia floral e sistema de reprodução de Jacquemontia multiflora (Choisy) Hallier f. (Convolvulaceae). Revista Brasileira de Botânica 23: 37–43. [Google Scholar]
- Pinheiro MCB, Ormond WT, Castro AC. 1991. Biologia da reprodução e fenologia de Zizyphus joazeiro Mart. (Rhamnaceae). Revista Brasileira de Biologia 51: 143–152. [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996.The natural history of pollination. London: Harper Collins Publishers. [Google Scholar]
- Quirino ZGM, Machado ICS. 2001. Biologia da polinização e da reprodução de três espécies de Combretum Loefl. (Combretaceae). Revista Brasileira de Botânica 24: 181–193. [Google Scholar]
- Ramirez N. 1989. Biología de polinización en una comunidad arbustiva tropical de la alta Guayana Venezolana. Biotropica 21: 319–330. [Google Scholar]
- Ramirez N, Gil C, Hokche O, Seres A, Brito Y. 1990. Biologia floral de una comunidad arbustiva tropical en la Guayana Venezolana. Annals of the Missouri Botanical Garden 77: 383–397. [Google Scholar]
- Ranta P, Blom T, Niemelä J, Joensuu E, Siitonen M. 1998. The fragmented Atlantic rain forest of Brazil: size, shape and distribution of forest fragments. Biodiversity and Conservation 7: 385–403. [Google Scholar]
- Rathcke BJ, Jules ES. 1993. Habitat fragmentation and plant–pollinator interactions. Current Science 65: 273–277. [Google Scholar]
- Real L. 1983.Pollination biology. Florida: Academic Press. [Google Scholar]
- Rebelo AG, Siegfried WR, Oliver EGH. 1985. Pollination syndromes of Erica species in the south-western Cape. South African Journal of Botany 51: 270–280. [Google Scholar]
- Renner S. 1998. Effects of habitat fragmentation on plant pollinator interactions in the tropics. In: Newbery DM, Prins HHT, Brown ND, eds. Dynamics of tropical communities. Oxford: Blackwell Science. [Google Scholar]
- Rodal MJN, Melo AL. 1999. Levantamento preliminar das espécies lenhosas da Caatinga de Pernambuco. In: Anais I Workshop Geral Programa Plantas do Nordeste. Kew: Royal Botanical Garden, 53–62. [Google Scholar]
- Rodal MJN, Andrade KVA, Sales MF, Gomes APS. 1998. Fitossociologia do componente lenhoso de um refúgio vegetacional no município de Buíque, Pernambuco. Revista Brasileira de Biologia 58: 517–526. [Google Scholar]
- Sazima M, Buzato S, Sazima I. 1999. Bat-pollinated flower assemblages and bat visitors at two Atlantic forest sites in Brazil. Annals of Botany 83: 705–712. [Google Scholar]
- Sampaio EVSB. 1995. Overview of the Brazilian Caatinga. In: Bullock SH, Mooney H, Medina E, eds. Seasonally dry tropical forests. Cambridge: Cambridge University Press, 35–63. [Google Scholar]
- Sampaio EVSB, Giulietti AM, Virginio J, Gamarra-Rojas CFL. 2002. Vegetação & flora da Caatinga. Recife: Associação Plantas do Nordeste – APNE; Centro Nordestino de Informações sobre plantas – CNIP. [Google Scholar]
- Saunders DA, Hobbs RJ, Margules CR. 1991. Biological consequences of ecosystem fragmentation: a review. Conservation Biology 5: 18–32. [Google Scholar]
- Sick H. 1997.Ornitologia brasileira. São Paulo: Editora Nova Fronteira. [Google Scholar]
- Silberbauer-Gottsberger I, Gottsberger G. 1988. A polinização de plantas do Cerrado. Revista Brasileira de Biologia 48: 651–663. [Google Scholar]
- Silva AG, Guedes-Bruni RR, Lima MPM. 1997. Sistemas sexuais e recursos florais do componente arbustivo-arbóreo em mata preservada na reserva ecológica de Macaé de Cima. In: Lima HC, Guedes-Bruni RR, orgs. Serra de Macaé de Cima: Diversidade Florística e Conservação em Mata Atlântica. Rio de Janeiro: Jardim Botânico, 187–211. [Google Scholar]
- SUDENE. 1990.Dados pluviométricos do Nordeste – Estado de Pernambuco. Série Pluviométrica 6. Recife: Superintendência do Desenvolvimento do Nordeste (SUDENE). [Google Scholar]
- Tabla VP, Bullock S. 2002. La polinización en la selva tropical de Chamela. In: Noguera FA, Rivera JHV, Aldrete ANG, Avendaño MQ, eds. História natural deChamela. Mexico: Intituto de Biologia-UNAM, 499–515. [Google Scholar]
- Vogel S. 1954.Blütenbiologische Typen als Elemente der Sippengliederung. Jena: Gustav Fischer Verlag. [Google Scholar]
- Vogel S. 1966. Parfümsammelnde Bienen als Bestäuber von Orchidaceen und Gloxinia Österreichischen Botanischen Zeitschrift 113: 302–361. [Google Scholar]
- Vogel S. 1968. Chiropterophilie in der neotropischen Flora. Neue Mitteilungen I. Flora 157: 562–602. [Google Scholar]
- Vogel S. 1974. Ölblumen und Ölsammelnde Bienen. Tropische und Subtropische Pflanzenwelt, 7. Wiesbaden: Steiner. [Google Scholar]
- Vogel S, Machado IC. 1991. Pollination of four sympatric species of Angelonia (Scroph.) by oil-collecting bees in NE Brazil. Plant Systematics and Evolution 178: 153–178. [Google Scholar]
- von Helversen O. 1993. Adaptations of flowers to the pollination by Glossphagine bats. In: Barthlott W, Naumann CM, Schmidt-Loske K, Schuchmann KL, eds. Animal–plant interactions in tropical environments. Bonn: Zoologisches Forschungsinstitut und Museum Alexander Koenige, 41–59. [Google Scholar]
- Waser NM. 1983. The adaptive nature of floral traits: ideas and evidence. In: Real L, ed. Pollination biology. New York: Academic Press, 241–285. [Google Scholar]