Abstract
Concentrations of Al, Si, Fe, Mn, Cu and Ca were analysed in leaves of ten Rubiaceae species, most of which are Al accumulators, and these were compared with concentrations in one species of Melastomataceae. Quantitative data confirmed the distribution of Al accumulation as previously determined by semi‐quantitative tests, and suggest that there is an apparent congruency between the shoot Al concentration and the number of accumulators within a certain genus or tribe. Al accumulators within the Rubiaceae are most characteristic of the Rubioideae subfamily, although a second origin is likely in at least a few members of the tribes Vanguerieae and Alberteae. While the leaf Si concentration in Melastomata malabathricum L. (Melastomataceae) was negligible, all Rubiaceae studied showed relatively high Si levels (mostly >3000 mg kg–1). It is hypothesized that an Al–Si complex is formed in the shoot tissues of Al‐accumulating Rubiaceae and that this may contribute to Al detoxification. However, the Si : Al mole ratio tended to differ widely among species. There was no significant correlation between Al and the other metals analysed. A remarkably high Mn concentration was found in Coptosapelta olaciformis Elm.
Key words: Accumulation, aluminium, Melastomataceae, metals, phylogeny, Rubiaceae, silicon
INTRODUCTION
Most plant species that grow on acid soils with a high level of soluble aluminium (Al) compounds have developed a wide range of resistance mechanisms to avoid or tolerate the toxic effects of Al (Foy et al., 1978; Roy et al., 1988; Kochian, 1995; Watanabe and Osaki, 2002). These plants can be grouped into two categories, Al accumulators and Al excluders, depending on whether or not Al is absorbed by roots in large quantities and is transported from the root to the shoot tissues. Most plants that have successfully adapted to acid soils follow the exclusion strategy and only a small number are Al accumulators (e.g. Haridasan, 1982; Haridasan and Araújo, 1988). These have been defined as plants that accumulate more than 1000 mg kg–1 in their leaf dry matter growing in a natural habitat (Chenery, 1948a, b; Jansen et al., 2002b).
Al accumulation raises interesting questions with respect to ecological and physiological features. One of the major environmental factors to which Al accumulation is related is soil acidity, since the level of bioavailable Al increases with decreasing pH. Interesting physiological problems deal, for instance, with Al transport, the speciation of Al forms, localization and detoxification mechanisms. To date, the exact metabolic role of Al in accumulating plants is unknown. This feature has also proved to be useful for evaluating phylogenetic relationships in plants at different taxonomic levels (Chenery and Metcalfe, 1983; Jansen et al., 2002a, b).
Chenery (1948b) suggested that the largest number of Al‐accumulating species belongs to the huge, mainly tropical, Rubiaceae family. Indeed, about 32 % of the total number of Al accumulators recorded belong to this family. Most Al‐accumulating species have been detected using semi‐quantitative spot‐tests. The ‘aluminon’ test devised by Chenery (1948a, b) is based on an ammonium aurine tricarboxylate reagent, which can be applied to both living and dried plant tissues: the slightly pink solution turns dark red/crimson depending on the amount of Al that has been accumulated. A similar spot‐test using a chrome azurol‐S reagent was employed to detect Al accumulation in wood (Kukachka and Miller, 1980). In earlier studies we applied these two tests to many members of the Rubiaceae and found that Al accumulation mainly characterized one of the three subfamilies, namely the Rubioideae (Jansen et al., 2000a, b). In addition, Al levels were suggested to be higher in leaves than in stem wood, but tissues of the bark were also found to show higher Al concentrations than those of the wood. However, precise quantitative data on Al are known for only a very limited number of Rubiaceae species.
Little is also known about exact levels of other metals in the Rubiaceae. A comparative study of the mineral composition of Al accumulators growing in a tropical rainforest in Indonesia suggested a relationship between Al and Si uptake in plants that accumulate more than 3000 mg kg–1 Al (Masunaga et al., 1998). Furthermore, the formation of an Al–Si complex was suggested in Faramea marginata Cham., an Al‐accumulating Rubiaceae species from the swamp forest in the Ilha de Mel (Brazil), but it is still not known whether this applies to other Al‐accumulating Rubiaceae (Britez et al., 1997, 2002). A comparative study of metal concentrations in this family may therefore provide further evidence on the Al–Si relationship, and may also illustrate whether or not additional metals are accumulated.
The aim of this paper is to determine exact Al concentrations in leaves of ten species of Rubiaceae, which are nearly all Al accumulators, using a precise quantitative method. Al levels are compared with results of previous spot‐tests and are evaluated in a phylogenetic framework. Furthermore, concentrations of other elements, in particular those of Si, Cu, Zn, Fe, Mn and Ca, have been measured to explore any correlation between these elements and Al. We also include in our analyses leaf samples of Melastoma malabathricum L., which is an Al‐accumulating member of the Melastomataceae. This family includes the second largest number of Al‐accumulating species in angiosperms (Jansen et al., 2002a). Data on Melastoma allow us to compare metal concentrations of Al and Si in two different families. While Si may play a role in Al detoxification in Rubiaceae, a different mechanism has been suggested for Melastomataceae (Watanabe et al., 1998).
MATERIALS AND METHODS
Dried leaves of 11 specimens from ten genera of Rubiaceae were collected at the herbarium of the National Botanic Garden of Belgium (BR) to obtain >0·2 g dry weight for each sample. Since there is generally no evidence of intraspecific variation in Al accumulation within Rubiaceae species growing in their natural habitat, we tested only one sample for ten species. However, two specimens of Emmeorhiza umbellata K.Schum. were included, which are indicated in the text as E. umbellata1 and E. umbellata2 (see below).
For the mineral analysis, 200 mg portions of the samples were digested using an acid mixture (HNO3 : HClO4 : H2SO4, 5 : 2 : 1). Concentrations of Al, Fe, Mn, Zn, Cu and Ca were determined by atomic absorption spectrophotometry (Z‐8000; Hitachi, Tokyo, Japan). Total Si values were calculated by analysing separately the concentrations of acid‐insoluble Si (SiO2) and acid‐soluble Si, which was taken up in the acid mixture. Concentrations of SiO2 (crude silica) were estimated by weighing the residue in the digested solution; the acid‐soluble concentration of Si was measured by Graphite Furnace Atomic Absorption Spectrometry (GFAAS). Mineral concentrations in Melastoma malabathricum L. (Melastomataceae) were obtained from seedlings grown in a nutrient solution with 0·4 mm Al and 0·65 mm Si (pH 3·8) (Watanabe et al., 1997; unpubl. res.).
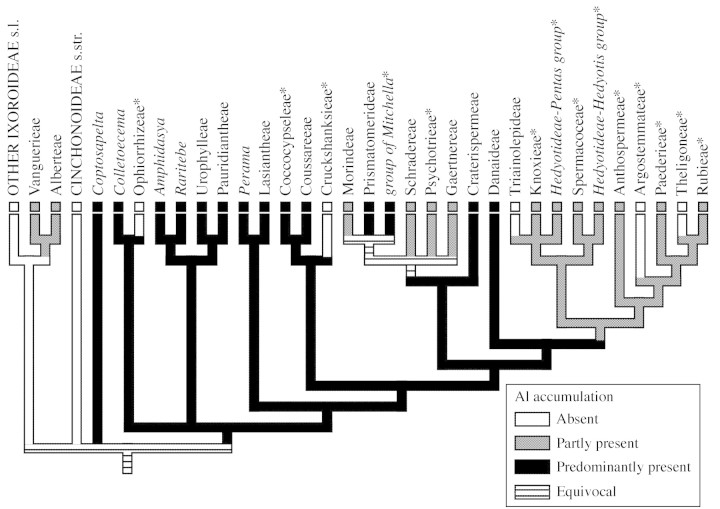
Without conducting a new phylogenetic analysis we have manually constructed a hypothetical tree that mainly summarizes molecular phylogenies (Bremer and Thulin, 1998; Andersson and Rova, 1999; Bremer et al., 1999; Bremer and Manen, 2000; Piesschaert et al., 2000). The distribution of Al accumulators was plotted on this hypothetical tree using MacClade 4 (Maddison and Maddison, 2000; Fig. 1).
Fig. 1. The distribution of Al accumulation plotted on a hypothetical tree based on Bremer and Thulin (1998), Andersson and Rova (1999), Bremer et al. (1999), Bremer and Manen (2000) and Piesschaert et al. (2000); data on Al accumulation from Jansen et al. (2000a, b). Taxa within the Rubioideae that are partly or entirely herbaceous are indicated by an asterisk.
The specimens studied, with details of their origin and collector (if known), are given in Table .
Table 1.
Details of the species studied
| Species | Origin | Collector and collection number |
| Alberta minor Baillon | Madagascar | A.M. Homolle s.n. (BR‐S.P. 834443) |
| Canthium confertum Korth. | Borneo, Tawao, Elphinstone Province | A.D.E. Elmer 20614 |
| Coccocypselum canescens Willd. ex Roem. & Schult. | Brazil, Est. Rio. R. de Sul, Kappesberg p. Montenegro | B. Rambo 47236 |
| Coptosapelta olaciformis Elm. | Philippine Islands, Surigao | C.A. Wenzel 3047 |
| Craterispermum laurinum Benth. | Liberia, approx. 10 miles north of Monrovia, savannah area | J.W.A. Jansen 1611 |
| Danais fragrans Pers. | Madagascar, Toliara, Intégral Réserve nr. 11, Andohahela, Parcelle 1, | B. Randriamampionona 334 |
| Emmeorhiza umbellata K.Schum. | ||
| E. umbellata1 | Brazil | C.F.P. von Martius (BR‐S.P. 802367) |
| E. umbellata 2 | Brazil | L. Riedel 160 |
| Gouldia terminalis (Hook. & Arn.) Hillebr. var. coriacea (Hook. & Arn.) Fosberg | Honolulu, Oahu, Manoa‐Palolo | (BR‐S.P. 810219) |
| Melastoma malabathricum L. | Plants cultivated from seedsin a glasshouse at the Laboratory of Plant Nutrition (Hokkaido University, Japan) | |
| Saprosma arboreum Blume | Borneo, Tawao, Elphinstone Province | A.D.E. Elmer 21452 |
| Triainolepis hildebrandtii Vatke | Tanzania, Rufiji, Mafia Island | H.J. Schlieben 2363 |
RESULTS
Quantitative data on Al, Si, Mn, Fe, Zn, Cu and Ca concentrations are given in Table for all specimens examined. There was significant variation in the concentration of Al and Si among the Rubiaceae specimens analysed. Triainolepis hildebrandtii was the only species in which the Al content was very low and could not be quantified exactly. All other species showed an Al concentration above 1000 mg kg–1. Levels of Al exceeding 9000 mg kg–1 were found in species of Coccocypselum, Coptosapelta, Craterispermum, Danais and Saprosma, while representatives of Alberta, Canthium, Emmeorhiza and Gouldia accumulated lower levels of Al. By far the highest Al concentration was found in Craterispermum laurinum (36 202 mg kg–1).
Table 2.
Leaf concentrations of elements and mole ratio of Si : Al and Si : Ca in ten Rubiaceae and one Melastomataceae species
| Family | Leaf concentrations (mg kg–1) | Mole ratio | |||||||
| Species | Al | Si | Mn | Fe | Zn | Cu | Ca | Si : Al | Si : Ca |
| Rubiaceae | |||||||||
| Alberta minor | 2472 | 586 | 92 | 61 | 17 | 1 | 2066 | 0·226 | 0·404 |
| Canthium confertum | 1885 | 6589 | 14 | 77 | 54 | 3 | 23 482 | 3·360 | 0·400 |
| Coccocypselum canescens | 10 357 | 6515 | 149 | 746 | 59 | 8 | 16 975 | 0·603 | 0·548 |
| Coptosapelta olaciformis | 10 535 | 3560 | 3644 | 160 | 46 | 5 | 8428 | 0·324 | 0·603 |
| Craterispermum laurinum | 36 202 | 13 438 | 587 | 391 | 33 | 18 | 11 389 | 0·356 | 1·684 |
| Danais fragrans | 9146 | 12 100 | 358 | 101 | 28 | 6 | 10 743 | 1·270 | 1·608 |
| Emmeorhiza umbellata 1 | 1222 | 22 075 | 68 | 160 | 42 | 6 | 14 214 | 17·386 | 2·216 |
| Emmeorhiza umbellata 2 | 2452 | 4812 | 24 | 250 | 32 | 5 | 15 865 | 1·886 | 0·433 |
| Gouldia terminalis | 1946 | 5118 | 892 | 57 | 39 | 2 | 24 595 | 2·526 | 0·297 |
| Saprosma arboreum | 9854 | 3571 | 737 | 50 | 41 | 3 | 15 731 | 0·347 | 0·324 |
| Triainolepis hildebrandtii | (Trace) | 5305 | 7 | 46 | 17 | 3 | 25 245 | – | 0·300 |
| Melastomataceae | |||||||||
| Melastoma malabathricum | 7130 | (Trace) | 208 | 651 | 36 | 11 | 12 143 | – | – |
Only one sample was tested for each specimen.
Total Si levels (acid‐soluble + acid‐insoluble Si) among the Rubiaceae analysed ranged from 586 mg kg–1 in Alberta minor to 22 075 mg kg–1 in Emmeorhiza umbellata1, but concentrations in most species were between 3000 and 15 000 mg kg–1. Only trace levels of Si were found in Melastoma malabathricum. The Si : Al mole ratio differed widely, varying from 0·226 in Alberta minor to 3·360 in Canthium confertum, and an exceptionally high value of 17·386 was found in Emmeorhiza umbellata1 (Table ).
Considerable variation also occurred with respect to concentrations of Mn, Fe and Ca, while relatively little variation was found for Cu and Zn. The concentration of Mn was surprisingly high in Coptosapelta olaciformis (3644 mg kg–1). Relatively high levels of Mn were also found in Gouldia terminalis (892 mg kg–1) and Saprosma arboreum (737 mg kg–1). Similar differences were found for Fe concentrations, since Coccocypselum canescens (746 mg kg–1) and, to a lesser extent, Craterispermum laurinum and Emmeorhiza umbellata2, showed relatively high levels of Fe. However, these quantities were not many times above the average quantity of Fe. Ca levels varied from 2066 mg kg–1 in Alberta minor to 25 245 mg kg–1 in Triainolepis hildebrandtii, and were frequently above 10 000 mg kg–1. The Si : Ca mole ratio was greater than 1 in Craterispermum laurinum, Danais fragrans and Emmeorhiza umbellata1.
DISCUSSION
Comparison of Al concentrations in Rubiaceae with results of earlier studies
The present data agree very well with earlier semi‐quantitative tests on Al concentrations in Rubiaceae. The ten Rubiaceae specimens studied accumulated Al in their leaves, i.e. contained Al levels above 1000 mg kg–1, whereas Triainolepis hildebrandtii showed no accumulation. In general, the number of accumulating species or genera appears to be a good indicator of the Al concentration in that group (Jansen et al., 2000a, 2002b). Hence, the feature tends to be most strongly expressed in taxa that consistently show positive spot‐tests for leaves and wood. A comparison of previous tests on leaves and wood with the new Al‐analyses is given in Table . In this table specimens are divided into three arbitrary groups according to their ability to accumulate Al: (1) strong Al accumulators (Al concentration >9000 mg kg–1); (2) intermediate accumulators; and (3) non‐accumulators (Al concentration <1000 mg kg–1). It is significant that the specimens categorized as strong accumulators almost always test positive in leaf‐tests according to Chenery (1946, 1948a, b) and Jansen et al. (2000b). The only exceptions are a specimen of Danais and one of Coccocypselum (see Table ). However, the single specimen of Coccocypselum that was found to give a negative result was cultivated in a glasshouse, and the lack of high Al levels in this specimen is probably due to the rather neutral soil conditions (Jansen et al., 2000b). Furthermore, the genera that are considered to be strong Al accumulators in Table nearly always test positive in the chrome azurol‐S test of wood samples.
Table 3.
Comparison of results of previous leaf‐ and wood‐tests with Al concentration reported in this paper
| Genus | Taxonomic position | Al‐test on leaves | Chrome azurol‐S test on wood | Al concentration (mg kg–1) in the specimen tested in this study |
| Strong Al accumulators | ||||
| Coccocypselum | Coccocypseleae, R | (15/15)1, 2, 3 (3/4)5 | – | 10 357 |
| Coptosapelta | Basal Rubioideae? | (7/7)3 (2/2)5 | (0/1)7 | 10 535 |
| Craterispermum | Craterispermeae, R | (9/9)2, 3 (3/3)5 | (4/4)6, 7 | 36 202 |
| Danais | Danaideae, R | (17/18)3 (2/2)5 | (3/4)7 | 9146 |
| Saprosma | Paederieae, R | (9/9)1, 2, 3 (2/2)5 | (2/2)7 | 9854 |
| Intermediate Al accumulators | ||||
| Alberta | Alberteae, I | (2/2)3 (4/6)5 | (0/2)7 | 2472 |
| Canthium | Vanguerieae, I | (14/25)3 (0/9)4 (1/3)5 | (0/1)7 | 1885 |
| Emmeorhiza 1 Emmeorhiza 2 | Spermacoceae, R | (1/1)3 (4/4)5 | (0/1)7 | 1222 |
| Gouldia | Rubioideae, R | (1/1)5 | 6 | 1946 |
| Non‐Al accumulator | ||||
| Triainolepis | Near Knoxieae, R | (0/1)3 (0/2)5 | (0/3)7 | Trace |
Data according to Chenery (1946)1, Chenery (1948a)2, Chenery (1948b)3, Webb (1954)4, Jansen et al. (2000a)5, Kukachka and Miller (1980)6 and Jansen et al. (2000b)7. In parentheses, the nominator gives the number of Al‐accumulating specimens, and the denominator the total number of specimens tested. R, Rubioideae; I, Ixoroideae.
The very high Al level in leaves of Craterispermum laurinum (36 202 mg kg–1) illustrates that this species is a strong Al accumulator. To the best of our knowledge, the highest level of Al found in a representative of the Rubiaceae is that reported by Chenery (1946) in Faramea insignis Standl. (40 000 mg kg–1), followed by Faramea anisocalyx Poepp. & Endl. (36 900 mg kg–1). Other members of the Rubiaceae that show Al levels above 10 000 mg kg–1 occur in the tribes Coussareeae, Craterispermeae, Lasiantheae, Pauridiantheae, Prismato merideae, Psychotrieae and Urophylleae.
The genera listed as intermediate or weak Al accumulators in Table show more variation in the semi‐quantitative tests. Leaf‐tests for some specimens of Alberta and Canthium were negative and, while those for Emmeorhiza and Gouldia were all positive, the number of specimens tested for Emmeorhiza was small and only a single sample of Gouldia was tested. Al concentrations in the specimens of these four genera range from 2472 to 1222 mg kg–1, and the chrome azurol‐S test is generally negative. This may suggest that Al is preferentially stored in leaves, and that Al accumulation in the secondary xylem occurs primarily when leaf levels are much higher than 1000 mg kg–1.
The absence of Al accumulation in Triainolepis hilde brandtii agrees with the exclusively negative leaf‐ and wood‐tests.
Phylogenetic aspects of Al accumulators in Rubiaceae
The distribution of Al accumulators is plotted on a hypothetical Rubiaceae tree that is based on molecular phylogenetic analyses (Fig. 1). Al accumulation tends to show two parallel origins within the family. While the majority of Al accumulators characterize the basal, predominantly woody, representatives of the Rubioideae, a second and independent origin is suggested within the Vanguerieae and Alberteae. However, Al levels in the latter two tribes appear to be lower than those in most of the Rubioideae. The Vanguerieae and Alberteae are closely related, as illustrated by molecular and morphological data (Andreasen et al., 1999). Within the Alberteae, Al accumulation is probably restricted to Alberta, because Nematostylis, the sole relative of Alberta, has been found to be non‐accumulating (Jansen et al., 2000b). Besides Canthium, the feature is also reported in at least one specimen of two other genera that belong to the Vanguerieae, namely Pachystigma and Perakanthus (Chenery, 1948a). Although the phylogenetic position of Coptosapelta needs further research, Al accumulation may indicate a position within or near the base of the Rubioideae (Jansen et al., 2002c).
The lack of Al accumulation is remarkable among the more derived tribes of the Rubioideae. This may be explained by these tribes’ tendency to herbaceousness. The influence of woodiness on Al accumulation can be illustrated by Saprosma, for example, which seems to show the highest Al levels in the Paederieae. Except for a slight positive ‘aluminon’ test of a specimen of Paederia foetida L., most representatives of the Paederieae are non‐accumulators (Jansen et al., 2000b). Saprosma has been transferred from the Psychotrieae to the Paederieae, and is the only (large) tree within the Paederieae; other members are shrubs or climbing shrubs (Puff, 1992). Nevertheless, it is still unclear why herbaceous plants generally do not accumulate Al. The high Al concentrations in the herbaceous genus Coccocypselum (Coccocypseleae) are striking. Despite its herbaceous habit, the presence of strong Al accumulation in Coccocypselum probably reflects the strongly supported, close relationship between the Coccocypseleae and the Coussareeae (see Fig. 1), which are suggested to include only species with very high Al levels (Britez et al., 2002).
Besides a clear taxonomic significance and considerable differences in Al levels between woody and herbaceous plants, the supply and uptake of Al in above‐ground plant tissues may also depend on the annual or perennial habit, as well as on the acidity, moisture and Al saturation of the soil. It appears that moderate or intermediate Al accumulators in the derived Rubioideae are perennial, whereas annual plants usually are non‐accumulators. However, perennial species are by no means always accumulators. Unfortunately, information about soil conditions is not available from the herbarium sheets of the material used in this study.
Si accumulation in Rubiaceae
In addition to Al accumulation, several Rubiaceae appear to accumulate Si. Plants that accumulate Si contain Si levels higher than 10 000 mg kg–1 and show Si : Ca mole ratios greater than 1 (Ma et al., 2001; Ma and Takahashi, 2002). Si accumulation therefore appears to be present in Craterispermum laurinum, Danais fragrans and Emmeorhiza umbellata1. Except for Alberta minor, all the Rubiaceae species analysed in this study had Si concentrations in excess of 1000 mg kg–1. In contrast, Si accumulation is absent in Melastoma malabathricum (Melastomataceae).
With respect to the strong Al accumulators, the trend is for a positive correlation between Al and Si concentrations, as a result of the high Al and Si levels in Craterispermum laurinum. However, this correlation is weakened when all the Rubiaceae analysed are considered, owing to the very high Si level and relatively low Al level in Emmeorhiza umbellata1. The high Si variation encountered in the two specimens of E. umbellata is striking. A relatively high Si concentration also occurs in Triainolepis hildebrandtii, but Al accumulation is absent in this species. Therefore, the present results do not indicate a strong correlation between Al and Si. Further analyses of a large number of non‐accumulating and accumulating species are needed, and the intraspecific variation should also be considered when interpreting Al–Si relationships in Rubiaceae.
There appears to be an interesting difference in the Si : Al mole ratio between strong and intermediate Al accumulators: strong accumulators generally show values of 0·3–0·6, whereas the Si : Al mole ratio is usually above 1 in intermediate Al accumulators. Exceptions are Danais fragrans (1·270) and Alberta minor (0·226), which are considered to be strong and intermediate Al accumulators, respectively. The Si : Al mole ratios observed in the strong Al accumulators correspond with those found in Faramea marginata Cham. (Rubiaceae, Coussareeae) (Britez et al., 2002). The Si : Al mole ratio in this species varies from 0·34 to 0·60, and it is suggested that more than one Al–Si compound is formed. Moreover, the mole ratio of Si : Al in F. marginata shows a significant positive correlation with Si concentration, but there is no correlation with Al concentration. Hence, Si seems to regulate Al accumulation in this species. Furthermore, the Si : Al mole ratio was found to be around 0·5 in Qualea grandiflora Mart. (Vochysiaceae), but around 0·1 in Q. parviflora Mart. (Vilarino, 2002). Thus, in Al accumulators from other families different Al–Si associations seem to be formed.
Al accumulation is thought to inhibit the uptake of high concentrations of Si because Al and Si are immobilized by forming complexes on the roots (Hodson and Evans, 1995). Moreover, it has been illustrated that Al‐silicate may help to detoxify the harmful effects of Al, but explanations for this mechanism are still controversial (Barcelo et al., 1993; Hodson and Sangster, 1993, 1999; Hodson and Evans, 1995; Cocker et al., 1998). Although the present results do not indicate a strong correlation between Al and Si concentrations, the high Si levels found may indicate that Al‐silicate is the primary form of Al in leaves of the Rubiaceae. This may reduce the toxic effects of Al, but other mechanisms may also contribute to Al detoxification. Al‐oxalate, however, has been reported to be the detoxified, chelated Al form in leaves of Melastoma malabathricum L. (Melastomataceae) (Watanabe et al., 1998).
Concentrations of other metals analysed
There was no significant correlation between concentrations of Al and those of other cations (Table ). Although a positive correlation between Al and Cu levels might be suggested because the highest concentrations of Cu and Al occur in Craterispermum laurinum, the limited variation in Cu concentration between strong and weak Al accumulators may indicate that this relationship is of little significance.
The variation found in Mn, Fe, Cu and Zn concentrations appears to be common when comparing different species within a native community. While normal levels of Mn fall within the range 20–500 mg kg–1 (Reeves and Baker, 2000), Mn accumulation (or ‘hyperaccumulation’ as for heavy metals) was defined as concentrations over 10 000 mg kg–1 (Baker and Brooks, 1989). Thus, despite its high level of Mn (3644 mg kg–1), Coptosapelta olaciformis cannot strictly be considered to be a hyperaccumulator. The principal report of plants showing high Mn concentrations was by Jaffré (1980), who studied species on the ultramafic soils in New Caledonia; the specimen of Coptosapelta studied here came from the Philippine Islands. It is unclear whether Coptosapelta may develop internal detoxification mechanisms for Mn in addition to Al detoxification. Other records of very high Mn concentrations in the Rubiaceae are not known. The relatively high content of Mn in Craterispermum laurinum, Gouldia terminalis and Saprosma arboreum may be explained by soil conditions.
Fe concentrations in plants are generally higher than Mn concentrations. Although there was considerable variation in Fe levels among the specimens analysed, accumulation of this element seems to be absent. None of the species studied showed Zn hyperaccumulation, with concentrations above 10 000 mg kg–1. Normal concentrations of Cu in plants are in the range 5–25 mg kg–1, but there have been occasional records of plants from copper‐mineralized areas, in particular the Katanga Copper Arc (Democratic Republic of Congo), with more than 1000 mg kg–1 Cu (Malaisse et al., 1994; Reeves and Baker, 2000). The majority of Cu‐accumulating plants has been reported from these areas in the Democratic Republic of Congo. A number of Rubiaceae genera, such as Otiophora, Manostachya, Pentanisia and Fadogia, are reported to grow in copper soils, but it is unclear whether hyperaccumulation of Cu is present in these species (Duvigneaud and Denaeyer‐De Smet, 1963; Wild, 1978).
ACKNOWLEDGEMENTS
We thank Drs M. Haridasan (Universidade de Brasília) and M. Broadley (HRI, Wellesbourne) for useful comments. Research at the Laboratory of Plant Systematics is supported by grants from the Research Council of the K.U. Leuven (OT/01/25) and the Fund for Scientific Research— Flanders (Belgium) (G.104·01). S.J. is a postdoctoral fellow and S.D. a research assistant of the Fund for Scientific Research—Flanders (Belgium) (F.W.O.‐Vlaanderen). T.W. is a Domestic Research Fellow of the Japan Society for the Promotion of Science (JSPS).
Supplementary Material
Received: 30 September 2002; Returned for revision: 22 November 2002; Accepted: 28 January 2003 Published electronically: 6 March 2003
References
- AnderssonL, Rova JHE.1999. The rps16 intron and the phylogeny of the Rubioideae (Rubiaceae). Plant Systematics and Evolution 214: 161–186. [Google Scholar]
- AndreasenK, Baldwin BG, Bremer B.1999. Phylogenetic utility of the nuclear rDNA ITS region in subfamily Ixoroideae (Rubiaceae): comparisons with cpDNA rbcL sequence data. Plant Systematics and Evolution 217: 119–135. [Google Scholar]
- BakerAJM, Brooks RR.1989. Terrestrial higher plants which hyperaccumulate metallic elements – a review of their distribution, ecology and phytochemistry. Biorecovery 1: 81–126. [Google Scholar]
- BarceloJ, Guevara P, Poschenreider C.1993. Silicon amelioration of aluminium toxicity in teosinte (Zea mays L. ssp. mexicana). Plant and Soil 154: 249–255. [Google Scholar]
- BremerB, Manen J‐F.2000. Phylogeny and classification of the subfamily Rubioideae (Rubiaceae). Plant Systematics and Evolution 225: 43–72. [Google Scholar]
- BremerB, Thulin M.1998. Collapse of Isertieae, re‐establishment of Mussaendeae, and a new genus of Sabiceeae (Rubiaceae); phylogenetic relationships based on rbcL data. Plant Systematics and Evolution 211: 71–92. [Google Scholar]
- BremerB, Jansen RK, Oxelman B, Backlund M, Lantz H, Ki‐Joong K.1999. More characters or more taxa for a robust phylogeny – case study from the coffee family (Rubiaceae). Systematic Biology 48: 413–435. [DOI] [PubMed] [Google Scholar]
- BritezRM, Watanabe T, Jansen S, Reissmann CB, Osaki, M.2002. The relationship between aluminium and silicon accumulation in leaves of Faramea marginata (Rubiaceae). New Phytologist 156: 445–456. [DOI] [PubMed] [Google Scholar]
- BritezRM, Reismann CB, Silva SM, Athayde SF, Lima RX, de Quadros RMB.1997. Chemical characterization of two forests on the coastal plain of the Ilha do Mel, Paraná, Brazil. In: Ando T, Fujita K, Mae T, Matsumoto H, Mori S, Sekiya J, eds. Plant nutrition – for sustainable food production and environment Dordrecht: Kluwer Academic Publishers, 461–462. [Google Scholar]
- CheneryEM.1946. Are Hydrangea flowers unique? Nature 158: 240–241. [Google Scholar]
- CheneryEM.1948a Aluminium in plants and its relation to plant pigments. Annals of Botany 12: 121–136. [Google Scholar]
- CheneryEM.1948b Aluminium in the plant world. Part I, General survey in dicotyledons. Kew Bulletin 1948: 173–183. [Google Scholar]
- CheneryEM, Metcalfe R.1983. Aluminium accumulation as a taxonomic character. In: Metcalfe CR, Chalk L, eds. Anatomy of the dicotyledons, Vol. II. Wood structure and conclusion of the general introduction Oxford: Clarendon Press, 165–167. [Google Scholar]
- CockerKM, Evand DE, Hodson MJ.1998. The amelioration of aluminium toxicity by silicon in higher plants: solution chemistry or an in planta mechanism? Physiologia Plantarum 104: 608–614. [Google Scholar]
- DuvigneaudP, Denaeyer‐De Smet S.1963. Cuivre et végétation au Katanga. Bulletin de la Société Royale Botanique de Belgique 96: 93–231. [Google Scholar]
- FoyCD, Chaney RL, White, MC.1978. The physiology of metal toxicity in plants. Annual Review of Plant Physiology 29: 511–566. [Google Scholar]
- HaridasanM.1982. Aluminium accumulation by some cerrado native species of central Brazil. Plant and Soil 65: 265–273. [Google Scholar]
- HaridasanM, de Araújo GM.1988. Aluminium‐accumulating species in two forest communities in the cerrado region of central Brazil. Forest Ecology and Management 24: 15–26. [Google Scholar]
- HodsonMJ, Evans DE.1995. Aluminium/silicon interactions in higher plants. Journal of Experimental Botany 46: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HodsonMJ, Sangster AG.1993. The interaction between silicon and aluminium in Sorghum bicolor (L.) Moench: growth analysis and X‐ray microanalysis. Annals of Botany 72: 389–400. [Google Scholar]
- HodsonMJ, Sangster AG.1999. Aluminium/silicon interactions in conifers. Journal of InorganicBiochemistry 76: 89–98. [Google Scholar]
- JaffréT.1980. Etude écologique du peuplement végétale des sols dérivés de roches ultramafiques en Nouvelle Calédonie. Vol. 124. Paris: Travaux et documents de l’ORSTOM. [Google Scholar]
- JansenS, Watanabe T, Smets E.2002a Aluminium accumulation in leaves of 127 species in Melastomataceae, with comments on the order Myrtales. Annals of Botany 90: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JansenS, Robbrecht E, Beeckman H, Smets E.2000a Aluminium accumulation in Rubiaceae: an additional character for the delimitation of the subfamily Rubioideae? International Association of Wood Anatomists Journal 21: 197–212. [Google Scholar]
- JansenS, Broadley M, Robbrecht E, Smets E.2002b Aluminium hyperaccumulation in angiosperms: a review of its phylogenetic significance. Botanical Review 68: 235–269. [Google Scholar]
- JansenS, Robbrecht E, Beeckman H, Smets E.2002c A survey of the systematic wood anatomy of the Rubiaceae. International Association of Wood Anatomists Journal 23: 1–67. [Google Scholar]
- JansenS, Dessein S, Piesschaert F, Robbrecht E, Smets E.2000b Aluminium accumulation in leaves of Rubiaceae: systematic and phylogenetic implications. Annals of Botany 85: 91–101. [Google Scholar]
- KochianLV.1995. Cellular mechanisms of aluminum toxicity and resistance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 46: 237–260. [Google Scholar]
- KukachkaBF, Miller RB.1980. A chemical spot‐test for aluminium and its value in wood identification. International Association of Wood Anatomists Bulletin 1: 104–109. [Google Scholar]
- MaJF, Takahashi E.2002. Soil, fertilizer, and plant silicon research in Japan. Amsterdam: Elsevier. [Google Scholar]
- MaJF, Miyake Y, Takahashi E.2001. Silicon as a beneficial element for crop plants. In: Datnoff LE, Snyder GH, Korndorfer GH, eds. Silicon in agriculture Amsterdam: Elsevier, 17–39. [Google Scholar]
- MaddisonWP, Maddison DR.2000. MacClade: interactive analysis of phylogeny and character evolution, version 4. Sunderland Massachusetts: Sinauer Associates Inc. [Google Scholar]
- MalaisseF, Brooks RR, Baker AJM.1994. Diversity of vegetation communities in relation to soil heavy metal content at the Shinkolobwe copper/cobalt/uranium mineralization, Upper Shaba, Zaïre. Belgian Journal of Botany 127: 3–16. [Google Scholar]
- MasunagaT, Kubota D, Hotta M, Wakatsuki T.1998. Mineral composition of leaves and bark in aluminium accumulators in a tropical rain forest in Indonesia. Soil Science and Plant Nutrition 44: 347–358. [Google Scholar]
- PiesschaertF, Andersson L, Jansen S, Dessein S, Robbrecht E, Smets E.2000. Searching for the taxonomic position of the African genus Colletoecema (Rubiaceae): morphology and anatomy compared to a rps16‐intron analysis of the Rubioideae. Canadian Journal of Botany 78: 288–304. [Google Scholar]
- PuffC.1992. On the correct tribal position of Saprosma Bl. (Rubiaceae). Second Flora Malesiana Symposium, Programme and Summaries: 34. [Google Scholar]
- ReevesRD, Baker AJM.2000. Metal‐accumulating plants. In: Raskin I, Ensley BD, eds. Phytoremediation of toxic metals: using plants to clean up the environment New York: John Wiley & Sons Inc. 193–229. [Google Scholar]
- RoyAK, Sharma A, Talukder G.1988. Some aspects of aluminum toxicity in plants. Botanical Review 54: 145–178. [Google Scholar]
- VilarinoC.R.O.2002. Acumulação de alumínio, calico e silício em espécies lenhosas do cerrado em dois solos. MSc Thesis, Universidade Federal de Uberlândia. [Google Scholar]
- WatanabeT, Osaki M.2002. Mechanisms of adaptation to high aluminum condition in native plant species growing in acid soils: a review. Communications in Soil Science and Plant Analysis 33: 1247–1260. [Google Scholar]
- WatanabeT, Osaki M, Tadano T.1997. Aluminum‐induced growth stimulation in relation to calcium, magnesium, and silicate nutrition in Melastoma malabathricum L. Soil Science and Plant Nutrition 43: 827–837. [Google Scholar]
- WatanabeT, Osaki M, Yoshihara T, Tadano T.1998. Distribution and chemical speciation of aluminum in the Al accumulator plant, Melastoma malabathricum L. Plant and Soil 201: 165–173. [Google Scholar]
- WebbLJ.1954. Aluminium accumulation in the Australian‐New Guinea flora. Australian Journal of Botany 2: 176–197. [Google Scholar]
- WildH.1978. The vegetation of heavy metals and other toxic soils. In: Werger MJA, ed. Biogeography and ecology of Southern Africa 2 The Hague: Dr W. Junk, 1303–1334. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.